Introduction
In mammals, hearing is defined as converting sound waves into electrical signals by two types of mechanotransducer hair cells, inner hair cell (IHC), and outer hair cell (OHC). These cells are comparable to 'tall' and 'short' hair cell in chicken cochlea (basilar papilla).
Medial olivocochlear (MOC) efferent neurons are located in the superior olivary complex and project via myelinated fibers to make synaptic contacts directly onto the base of OHCs. Before the onset of hearing, IHCs are transiently innervated by MOC efferents as well. Although gamma-aminobutyric acid, calcitonin gene related peptide, and opioid peptides are also present in MOC terminals, acetylcholine (ACh) is considered to be the primary transmitter of the efferents.1) ACh released by efferent terminals binds postsynaptically to the highly calcium permeable ╬▒9╬▒10 nicotinic acetylcholine receptors (nAChRs). This leads to the subsequent activation of calcium dependent SK K+ channels and K+ efflux, hyperpolarizing the hair cell (Fig. 1).2) Hyperpolarization of OHCs eventually induces inhibition of neural signals from afferent fibers and inner hair cells, thus efferent inhibition provides feedback and modulation of the afferent signals.3) Efferent inhibition offers the potential to improve signal detection in noisy environment, to selectively attend to particular signals, and to protect the cochlea from loud sound damage.
In this review, author provide the information about 1) mechanism of efferent inhibition, 2) basic information of electrophysiology such as patch clamp, 3) role of nAChRs on efferent inhibition, especially calcium permeability of receptor.
Mechanism of Efferent Inhibition
Efferent innervation of the cochlea
MOC efferent neurons are located in the superior olivary complex of the brainstem and project to the cochlea. Lateral olivocochlear efferent (LOC) originates from the lateral superior olivary nucleus, and projects to IHCs in the ipsilateral side. Efferent fibers are divided into the uncrossed and crossed olivocochlear bundles, with the latter crossing the midline near the floor of the fourth ventricle.4) Although both the LOC and MOC contain crossed (contralateral) and uncrossed (ipsilateral) fibers, in most mammalian species the majority of LOC fibers project to the ipsilateral cochlea, while the majority of the MOC fibers project to the contralateral cochlea.
Within two week from birth, the rat's MOC efferents transiently innervate IHCs prior to the onset of hearing.5) Thus, the recordings of IHCs and ACh puff stimulation of IHCs can mimic efferent response of OHCs in the early developmental period. IHCs of neonatal rats are useful for the efferent inhibition research, because of the easy dissection and transient innervation of MOC efferents. These efferent axosomatic synapses with immature IHCs are supposed to have a functional importance since, first, the efferent synapse on immature IHCs is functional6) and second, axosomatic synapses reappear transiently in adulthood after the IHC has been disconnected from auditory dendrites by excitotoxic injury.7) These results have suggested that the transient efferent innervation may play a role in the functional maturation and recovery of the cochlear hair cells.5)
Acetylcholine induces hyperpolarizing inhibitory synaptic potentials
Brainstem neurons project to4) and suppress the response of the cochlea to sound.8) These efferent neurons are cholinergic, and a considerable list of evidence supports the role of ACh as the efferent inhibitory transmitter.9-11) Intracellular recordings revealed that efferent activity caused hyperpolarizing inhibitory postsynaptic potentials (IPSPs) in hair cells.12) Application of ACh to hair cells isolated from the chick's cochlea causes a biphasic voltage change that results from the sequential activation of ligand-gated cation channels, followed by current through calcium-activated potassium channels. A novel nAChR mediates this response. The calcium-dependent potassium channels activated in hair cells by ACh (SK channel) are apamin-sensitive and of small conductance.13-16)
Efferent coupling in both nAChRs and calcium dependent SK K+ channels
Efferent neurons release ACh to inhibit sensory hair cells of the inner ear. As implied by their voltage-dependence, calcium entry through the nAChRs triggers calcium-dependent potassium current to hyperpolarize hair cells. Efferent activity induces long-lasting hyperpolarizing IPSPs in cochlear hair cells, and this efferent activity consists of a brief depolarization preceding a much larger, long-lasting hyperpolarization. Thus ACh causes a biphasic change in the membrane conductance of mammalian cochlear hair cells. Recent studies revealed that first inward current through nAChRs makes a brief depolarization, and next potassium outward current through SK channel makes a much larger, long-lasting hyperpolarization. Systematic coupling between nAChRs and calcium dependent SK K+ channels play an important role in efferent inhibition of cochlear hair cells (Fig. 1B).
Synaptic cistern as intracellular calcium store
Synaptic cistern, a near membrane endoplasmic reticulum, is co-extensive with the efferent synaptic contact. The internal calcium stores may participate in efferent inhibition. Prolonged ACh application induced the irregular, highly variable waveform of the calcium-induced calcium release (CICR). CICR is a form of sporadic calcium release from the synaptic cistern. In the surface of synaptic cistern, there are two major ion channels: one is sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA), and the other is a ryanodine receptor. SERCA calcium pump moves calcium from the cytoplasm to the synaptic cistern, and refills calcium into the synaptic cistern. Ryanodine receptor provides additional calcium from synaptic cistern to subsynaptic space, and evokes calcium-induced SK channel activation. Thus, this sporadic calcium release through ryanodine receptor is CICRs. An interesting feature of the synaptic cistern is the difference of width depending upon species. For example, extensive cisterna was shown in chick hair cells, and the narrow cisterna was observed in mammalian hair cells. However, functional study will be needed to reveal the difference of synaptic cistern between birds and mammals.
Basic Information of Electrophysiology Such as Patch Clamp
Recording of chick auditory organ (basilar papilla) and rat cochlea
We obtained patch-clamp recordings from the inner hair cells of the apical turns in the cochlea excised from young (P9-P10) rats, in which efferent synaptic input is commonly found.17,18) The auditory organ (basilar papilla) was dissected from the temporal bone of embryonic chickens (17-20 d in ovo). After 5-10 min of exposure to protease (Sigma-Aldrich Type XXIV, 0.1 mg/mL) in buffered saline, the tegmentum vasculosum and tectorial membrane were removed, exposing the sensory hair cells. The basilar papilla or cochlea was secured to a coverslip by small spring clips (made from fine insect pins) and transferred to a recording chamber and viewed with differential interference contrast using a 40├Ś water immersion objective and a camera with contrast enhancement (Fig. 2A).
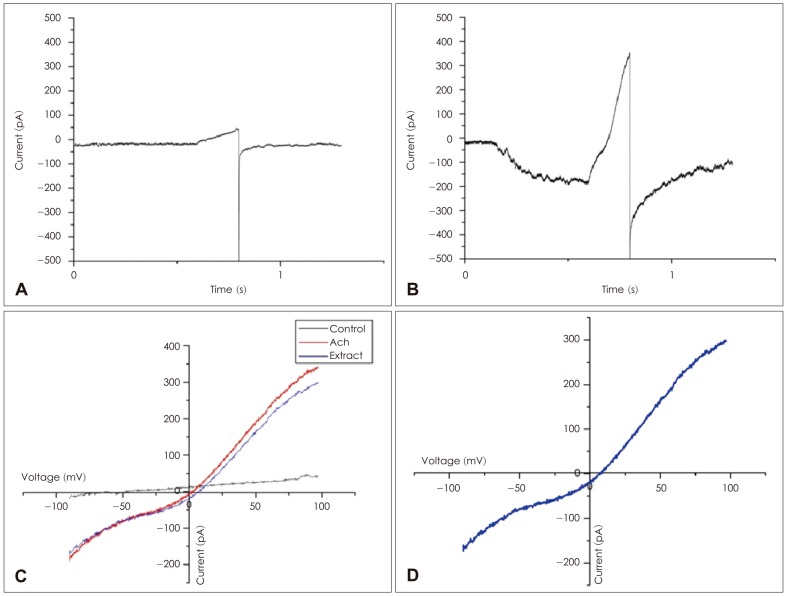
Short hair cells were recorded in a region corresponding to 500-1000 Hz, along the tonotopically organized basilar papilla of the chicken (0.25-0.5 mm, the distance from the apical tip toward the base). These cells were confirmed to be short hair cells by their position farthest from the neural limbus, where they receive predominantly efferent innervation.19) Finally, these cells responded to ACh with a combination of ligand-gated cation current, followed by Ca2+-dependent potassium current through apamin-sensitive ("SK-like") potassium channels, as demonstrated previously.20) Patch and puffer pipettes (3-5 MŌä”, coated with Sylgard silicon elastomer and fire-polished) were mounted on piezoelectric manipulators for positioning on the hair cells. Voltage-clamp recordings were obtained using an Axopatch 200B, running under Clampex software. Recordings were made at room temperature. Whole-cell, giga-ohm seal intracellular recordings were made on 'short' (abneural) hair cells approximately at the midpoint of the 4 mm long basilar papilla (chicken auditory organ) from late-stage embryos (E17-20) using intracellular cesium/BAPTA (10 mM) and extracellular apamin (300 nM) to minimize the SK current (Fig. 2B). Puff application of ACh (1 mM, 500 ms) elicited inward currents at negative membrane potentials that reversed near 0 mV, which means calcium dominant ion channel. The reversal potential was determined using 'ramp' voltage commands from -100 to +100 mV, 200 ms duration, designed to coincide with the steady maximal response to ACh. Control I-V values were subtracted from those obtained in the presence of ACh to measure the reversal potential of the ACh evoked current (Fig. 3).
Measurement of reversal potential and calcium permeability
To measure reversal potentials as a function of external calcium, we used an extracellular solution containing 100 mM NaCl, 5.8 mM KCl, 0.1-10 mM CaCl2, 5.6 mM d-glucose, and 10 mM Hepes buffer (pH 7.4), with osmolality adjusted to 300-320 mosmol/kg with sucrose. To measure current through the cationic nAChR only, without contaminating SK current, we used a pipette solution containing 140 mM CsCl, 3.5 mM MgCl2, 10 mM BAPTA-AM, 5 mM Hepes buffer, and 2.5 mM Na2ATP, with pH adjusted to 7.2 with CsOH. Apamin (300 nM) was added to the external saline to further minimize calcium-activated SK currents. The experiment was designed to study the voltage dependence of the cationic nAChR currents and/or the changes in the current reversal potential. Thus KCl was replaced by CsCl to eliminate voltage-dependent K+ currents.21)
The relative Ca2+ to monovalent permeability (pCa/pMono) was evaluated by analyzing the shift in the reversal potential (Erev) as a function of the increase in the extracellular Ca2+ concentration. Permeability ratios were calculated for each recorded cell and then averaged.
Role of Nicotinic Acetylcholine Receptors on Efferent Inhibition
Efferent cholinergic inhibition of mammalian and avian auditory hair cells is thought to be mediated by nAChRs. Gating of the nAChR leads to activation of calcium-dependent potassium channels to hyperpolarize the hair cell. Heterologous expression in Xenopus oocytes has shown that mammalian (rat) ╬▒9╬▒10 nAChRs have a substantial permeability to calcium (pCa/pNa -9) similar to that of the native nAChR (pCa/pNa -8).22) In contrast, avian (chicken) ╬▒9╬▒10 in oocytes is significantly less Ca2+-permeable (pCa/pNa <3), and here we provide a comparison with the properties of the native cell nAChR between the avian and mammalian species. In most cells, a standard voltage-step protocol also was used. These measurements (5-8 cells each) were carried out in 0.1, 1, and 3 mM calcium saline. The ACh-evoked current was largest in 1 mM calcium, and displayed sharp outward rectification. Outward rectification was more pronounced in higher calcium concentrations and could be reduced by conditioning depolarization, with both observations supporting a 'calcium permeation and block' effect. There was no significant effect of calcium on reversal potential for these concentrations, but I-V curves tend to show more positive Erev in higher external calcium (Fig. 4A). From the extended equation, the Ca2+ permeability relative to that of the dominant monovalent cation was calculated as 2.6. The reversal potential of ACh-evoked currents of rat inner hair cells had a steeper slope, and a calculated pCa/pMono of 8. Thus, like the recombinant avian ╬▒9╬▒10, the native chicken hair cell nAChR appears to have a relatively low permeability to calcium. In these results, the mammalian cochlear nAChRs are approximately 3 times more permeable to calcium compared with the avian nAChRs (Fig. 4B). Considering the similar functional power of efferent inhibition between the avian and mammalian species, relative contribution of synaptic cistern to activate SK channel as calcium provider may be different, and more powerful synaptic cistern of the avian species may compensate lower avian nAChRs permeability. Future study should focus on the functional measurement of synaptic cistern and other contributable factor to efferent inhibition.
Conclusion
1) Efferent inhibition: calcium influx (nAChR)+potassium outflux (SK channel).
2) Erev of avian nAChR moved to positive holding voltage according to Ca2+ concentration, but is much more flat than Erev of rat nAChR. This result shows that avian nAChR is less permeable to calcium than mammals.
3) Calcium permeation is more effective in nAChRs of mammalian cochlea than avian cochlea (about 3 times more in native cochlear hair cell).
4) In efferent system, main calcium providers to SK channel are nAChR and synaptic cistern, which contribution to efferent inhibition is different between avian and mammalian species.