Safety of Autologous Umbilical Cord Blood Therapy for Acquired Sensorineural Hearing Loss in Children
Article information
Abstract
Background and Objectives
Sensorineural hearing loss (SNHL) in children is associated with neurocognitive morbidity. The cause of SNHL is a loss of hair cells in the organ of Corti. There are currently no reparative treatments for SNHL. Numerous studies suggest that cord blood mononuclear cells (human umbilical cord blood, hUCB) allow at least partial restoration of SNHL by enabling repair of a damaged organ of Corti. Our objective is to determine if hUCB is a safe treatment for moderate to severe acquired SNHL in children.
Subjects and Methods
Eleven children aged 6 months to 6 years with moderate to severe acquired SNHL were treated with intravenous autologous hUCB. The cell dose ranged from 8 to 30 million cells/kg body weight. Safety was assessed by measuring systemic hemodynamics during hUCB infusion. Infusion-related toxicity was evaluated by measuring neurologic, hepatic, renal and pulmonary function before and after infusion. Auditory function, auditory verbal language assessments and MRI with diffusion tensor imaging (DTI) were obtained before and after treatment.
Results
All patients survived, and there were no adverse events. No infusionrelated changes in hemodynamics occurred. No infusion-related toxicity was recorded. Five subjects experienced a reduction in auditory brainstem response (ABR) thresholds. Four of those 5 subjects also experienced an improvement in cochlear nerve latencies. Comparison of MRI with DTI sequences obtained before and after treatment revealed increased fractional anisotropy in the primary auditory cortex in three of five subjects with reduced ABR thresholds. Statistically significant (p<0.05) reductions in ABR thresholds were identified.
Conclusions
TIntravenous hUCB is feasible and safe in children with SNHL.
Introduction
Sensorineural hearing loss (SNHL) is a permanent sensory disorder affecting more than 270 million people worldwide. The incidence of SNHL increases from 2/1000 in newborns to 5/1000 in children aged 3-17 years to 33% of adults aged 65-74 years to 50% of those greater than 85 years of age [1,2]. Existing treatments (hearing aids and cochlear implants) are designed to improve the symptoms of SNHL by augmenting the damaged organ of Corti. These treatments fail to reverse the underlying pathology, which is the loss of sensory inner hair cells in the organ of Corti. Inner, outer and structural hair cells are necessary for hearing and ultimately transform sound waves into electrical impulses transmitted to the brain. The loss of hair cells reduces auditory input to the brain, and hearing impairment develops with sufficient hair cell loss. In mammals, the organ of Corti is post-mitotic at birth, and no spontaneous hair cell regeneration occurs thereafter.
Among infants and children with SNHL, 23% to 50% of SNHL is the result of a genetic mutation (Connexin 26 mutation, Waardenburg syndrome, Usher syndrome, mitochondrial disorders, etc.) [2-5]. The remaining infants and children have acquired SNHL, which is most commonly attributed to prematurity, infection (in utero or post-delivery) and exposure to noise or oto-toxic drugs.
In preclinical and clinical studies, the intravascular delivery of mesenchymal progenitor cells in acute neuro-pathologic insults (stroke, traumatic brain injury, spinal cord injury, etc.) have shown significant promise [6-12]. Experimentally deafened mice and guinea pigs experienced cochlear repair after cord blood mononuclear cells (human umbilical cord blood, hUCB)-derived cell transplantation [13,14]. Following myeloablation and hUCB transplantation, patients with mucopolysaccharidosis have experienced an improvement in SNHL [15]. The most common cell populations used in these trials include bone marrow mononuclear fraction and hUCB mononuclear fraction. The bone marrow treatments require a bone marrow harvest. The hUCB treatment utilizes a mixed cell population rich in progenitor cells that are collected and cryopreserved at birth. Minimal cell processing is required, and an ample cell number is usually available for use in a pediatric population. Using an autologous cell product, we avoid the potential for graft vs host disease, and cell rejection and blood-borne disease transfer can be avoided. Acquired SNHL is a neuro-pathologic insult to the organ of Corti that may respond to hUCB treatment.
This study presents the results of a phase 1 clinical trial to evaluate the intravenous administration of hUCB mononuclear fraction in infants and children with acquired SNHL. Our protocol was designed to evaluate the feasibility, logistics and safety of autologous hUCB treatment in this patient population. We also sought to acquire limited dose/response data, auditory function, language development and structural [MRI with diffusion tensor imaging (DTI) sequences] outcome data for future trial planning.
Subjects and Methods
This study was conducted under Federal Investigational New Drug (IND) Application #15354 and was approved by the Florida Hospital Committee for the Protection of Human Subjects (IRBnet #434269) and Florida Hospital Office of Research Administration.
Patient enrollment
Following IND and IRB approval, a recruitment email was sent to families who had banked their children’s cord blood with Cord Blood Registry® (CBR®, San Bruno, CA, USA). The email invited parents of children with SNHL to contact the research team and the Florida Hospital for Children. After contact was initiated, an informed consent form was sent to the subject’s parents/guardians. Once the parents/guardians had reviewed, signed and returned the informed consent, a telephone interview was performed with a member of the research team. Following the interview, parents/guardians who wished to participate forwarded their child’s medical records to Orlando for review. If the subject met the inclusion and exclusion criteria (Table 1), human leukocyte antigen (HLA) typing was performed on the potential study subject and then compared to the results from a sample received from CBR® for identity confirmation. If the HLA typing matched, arrangements were made for the subject’s hUCB unit to be sent to the Florida Hospital Cell Processing Lab where it remained stored in liquid nitrogen until the date of infusion. A treatment date was then arranged with the subject’s parents/caregivers, and travel plans were confirmed.
Patients could only be enrolled longitudinally after review of their initial post-treatment course by an independent data safety management board. All data were audited by an external clinical monitor. Fig. 1 is a graphic timeline of the patient enrollment and treatment experience.
Patient management
Parental informed consent was re-obtained in person prior to obtaining a history, conducting a physical exam and performing baseline audiological and neurological assessments. Auditory verbal speech language assessment, audiology testing [auditory brainstem response (ABR), otoacoustic emission testing (OAE), tympanometry and audiogram], screening laboratory and X-ray testing were also performed at the baseline visit.
On the day of infusion, the patient was admitted to the Florida Hospital for Children. Under general anesthesia, a 3 tesla MRI of the brain with DTI sequences was obtained. If the nonsedated ABR was inadequate, an ABR was obtained under anesthesia. The patient was allowed to recover from anesthesia and was then admitted to the bone marrow transplant unit of the Florida Hospital for Children for continued recovery and monitoring.
Cord blood infusion
On the day of the infusion, the hUCB unit was thawed and washed in the Florida Hospital Cell Processing Laboratory. When the cord blood preparation was completed and the cord blood unit was determined to have met laboratory release criteria, it was transported to the bone marrow transplant unit. After baseline vital signs were obtained, the subjects were pre-medicated with diphenhydramine (Benadryl, Johnson & Johnson, New Brunswick, NJ, USA) and methylprednisolone (Solu-Medrol, Pfizer, New York, NY, USA). Thirty minutes after premedication, the hUCB unit was infused intravenously under the supervision of a hematologist (DS) experienced in bone marrow transplantation. Infusion was gravity-based. Vital signs were recorded every 15 minutes during the infusion, as well as for 4 hours following infusion. The patient was monitored overnight in the bone marrow transplant unit, and baseline laboratory values as well as a chest X-ray were rechecked in the morning following infusions. If the repeat lab values and chest films were within expected limits, the patient was discharged from the hospital. The parents/caregivers were provided with a digital thermometer and were contacted daily to follow the patient’s status for 14 days following discharge. The patient’s parents/caregivers were instructed to follow-up with their primary care physician on returning home.
Follow-up visits
The subjects and their parents returned for follow-up at 1, 6 and 12 months post infusion. At the 1-month, 6-month and 12-month follow-up visits, physical and neurological examinations were performed as well as tympanometry, OAE and ABR testing. Speech-language pathology evaluations were performed during the 6 and 12-month follow-up visits. At the 12-month follow-up visits, an MRI with DTI sequences was obtained.
Monitoring for infusion-related toxicity
Pulmonary
Pulmonary function was assessed by continuous PaO2 measurement during anesthesia for the MRI/ABR, and while the patient was hospitalized in the bone marrow transplant unit for hUCB infusion. PaO2 was also recorded at each follow-up visit. Standard chest X-rays were obtained on the day of hUCB infusion, the day after hUCB infusion and at each follow-up visit.
Renal
Serum creatinine and blood urea nitrogen were obtained as a measure of renal function before hUCB infusion, the day after hUCB and at each follow-up visit.
Neurological
A complete neurological examination was performed one day prior to and one day following hUCB infusion and at each subsequent follow-up visit.
Hematologic
A complete blood count with differential and platelet count was obtained one day before hUCB infusion, the day after hUCB infusion and at each follow-up visit.
Hepatic
The hepatic transaminases [aspartate transaminase (AST) and alanine transaminase (ALT)] were measured the day before hUCB infusion, the day after hUCB infusion and at each follow-up visit as an index of hepatic injury/toxicity.
Longitudinal functional and language outcome testing
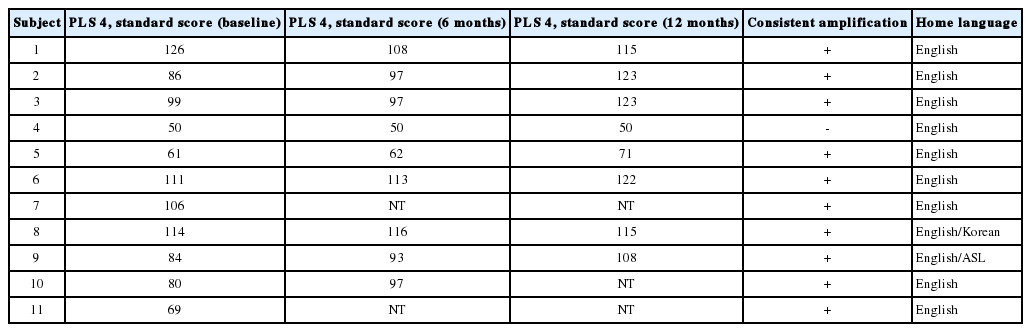
ABR, OAE, audiogram and tympanometry were obtained on the day before or if necessary on the day of the hUCB infusion and at each subsequent follow-up visit. Audiologic data were reviewed at the time of collection by a senior audiologist (EM). Changes of greater than a ±5 dB ABR threshold were considered significant. Changes greater than ±0.5 milliseconds in peak 5 of CN VIII conduction latency were considered significant. Age-appropriate speech-language pathology testing was obtained by an experienced auditory verbal speechlanguage pathologist (LB) on the day before hUCB infusion and at the 6- and 12-month follow-up visits, using the Preschool Language Scale, 4th edition [16].
Statistical methods
Statistical analysis was performed by a biostatistician (RN) experienced in analyzing audiologic data. SAS 9.4 (Statistical Analysis System, Cary, NC, USA) was used to perform all the analyses. Statistical significance tests were performed for the effect of hUCB infusion on ABR thresholds and CN VIII peak 5 latencies using the change scores at the three follow-ups after the intervention. To assess the efficacy of the treatment, within-subject analysis of variance (ANOVA) was performed for the change scores at the 1-month, 6- month and 12-month follow-ups. For the ABR thresholds, 5 different measurements at various frequencies [click 2,000 Hz, tone burst (TB) at 500, 1,000, 2,000 and 4,000 Hz] were recorded for each ear, resulting in a total of 10 measurements for each subject at each follow-up time point. For latency, 6 different measurements at various frequencies were recorded for each ear, resulting in a total of 12 measurement for each subject at each follow-up time point. Apart from investigating the overall treatment effect, we also studied the direction of change in the outcome for individual subjects. Due to the very small sample size as well as some missing data within the pool, the overall power is expected to be low. Consequently, statistical significance for treatment may not be achieved for all of the outcomes using ANOVA, even if the treatment is effective. However, even in the absence of statistical significance, if the change in the relevant outcome is in the expected direction in most cases, it can be considered to be an evidence towards efficacy of the treatment. Under the null hypothesis of no treatment effect, there is a 50% chance for the change in each outcome to be in the desired direction. The probability of observing a specific number of change scores in the desired direction can be easily calculated using a binomial distribution with probability 0.5. Let k be the number of observed change scores in the desired direction. If the probability of observing at least k change scores in the desired direction using binomial distribution is less than 0.05 under the null hypothesis, the result will be considered statistically significant and the treatment be deemed effective.
3-T MRI with DTI sequences
Imaging protocols for brain MRI with DTI were performed using 3.0-T MR (Verio; Siemens Medical, Erlangen, Germany) with a 16-channel head coil. The DTI sequence was as follows: time to echo 84 ms, time to relaxation 10,000 ms, 250×250 mm slice with 4 mm spacing, 0 mm gap, matrix 128×100, 38 directions 25, maximum B value 1,000 s/mm2. Images were obtained on the day of hUCB infusion and at the 12-month follow-up visit. DTI imaging was processed with DynaSuite (In-Vivo; Gainesville, FL, USA). Maximum and mean fractional anisotropy (FA) were recorded for bilateral inferior colliculi, medial geniculate, auditory radiations, and white matter of Heschl’s gyrus for each patient at baseline and 1-year followup MRI using region of interest (ROI) analysis. All images were reviewed in a blinded fashion by an experienced neuroradiologist (SM).
Results
Demographics
The clinical demographics of the patients enrolled in the study are summarized in Table 2. Subject enrollment began in November 2013, and follow-up evaluations were completed in February 2017. Subjects were enrolled longitudinally, allowing for a complete one-month safety review of each patient before enrolling the next subject.
Cord blood infusion hemodynamics
There were no significant hemodynamic changes during or after the hUCB infusion, nor was there a notable change in hemoglobin/hematocrit levels after the procedure.
Cell dose characterization
The total nucleated cells (TNC) administered, age at treatment and response to treatment are summarized in Table 3. Clinical improvements were only found following hUCB doses greater than 15×106 TNC/kg.
Infusion-related toxicity
Pulmonary
There were no significant changes in PaO2 between the pre-hUCB infusion, post-hUCB infusion or follow-up PaO2 measurements. No changes were detected on chest X-rays obtained before or after hUCB infusion or at any follow-up visits.
Renal
The serum creatinine and blood urea nitrogen levels remained stable and within normal limits before and after hUCB infusion as well as at all follow-up visits.
Hepatic
The ALT and AST transiently and mildly increased following hUCB infusion in 2/11 patients (subjects 5 and 10). Both patients were observed as inpatients for an additional 24 hours post infusion. On repeat lab testing, the ALT and AST had either normalized or were decreasing toward normal. The changes were typical of those observed following stem cell infusion for other conditions. The AST and ALT were within normal limits on all follow-up measurements.
Neurological
The neurological examinations remained stable for all subjects before and after hUCB infusion and at all follow-up visits.
Longitudinal functional and speech-language outcomes
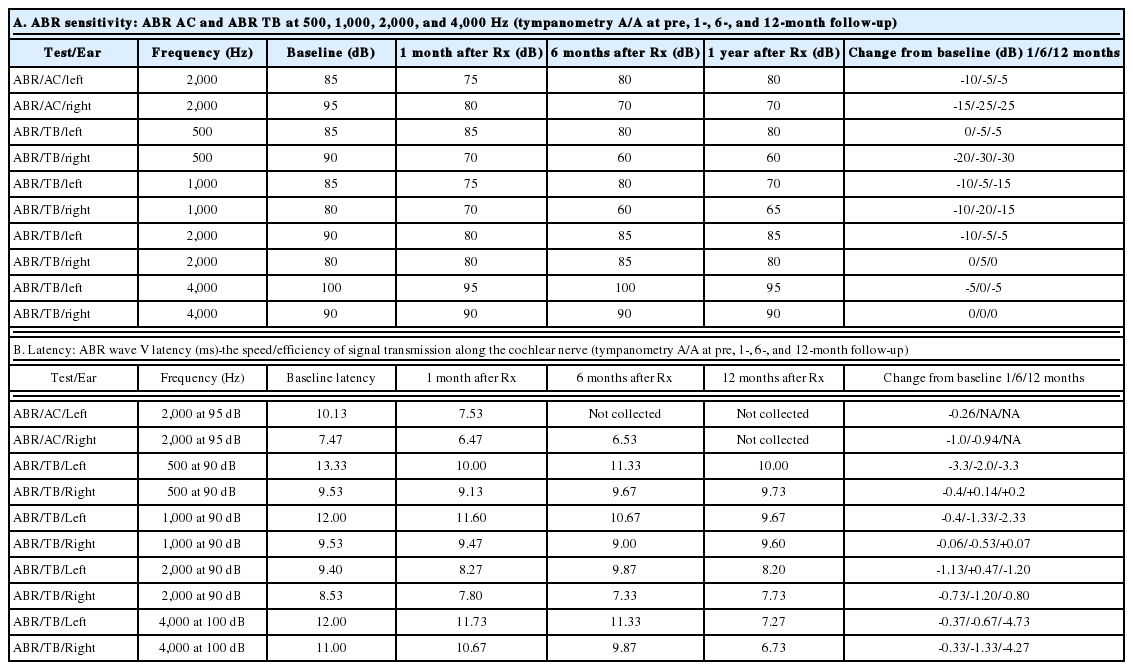
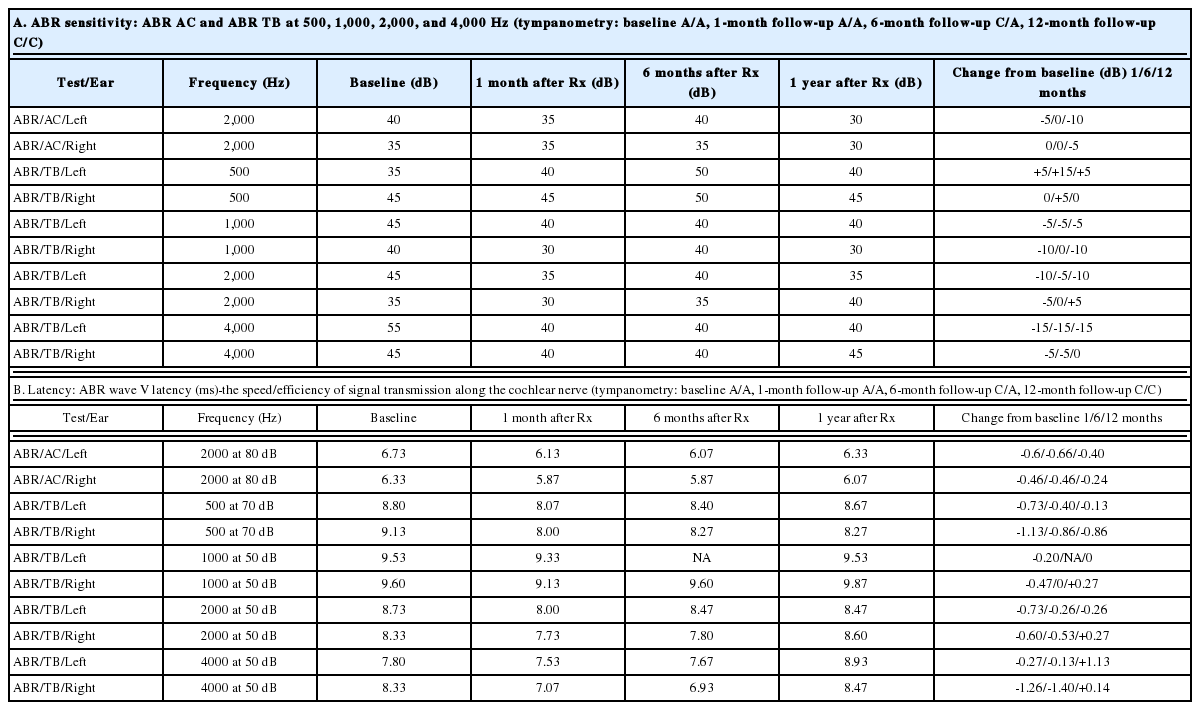
ABR threshold improvement was observed in five subjects. Four of these five subjects also experienced improvements in CN VIII peak 5 latencies. Representative pre- and post-treatment audiograms, as well as corresponding ABR tracings, are shown in Fig. 2. Data for the four subjects with both the ABR threshold and CN VIII peak 5 latency improvement are summarized in Table 4-7. When improvements in ABR threshold or latency occurred, they were evident on one-month follow-up testing and were durable throughout the study follow-up (Table 4-7). Subject 4, who experienced a reduction in ABR threshold without an improvement in CN VIII peak 5 latency, was the only child to experience worsening language scores (Table 8). In this instance, the parents revealed poor adherence to recommended hearing aid use and speechlanguage therapy. No 6- or 12-month follow-up data were obtained for Subject 11, whose parents chose to proceed with bilateral cochlear implantation after he did not experience any improvements at the one-month follow up testing. While speculative, reductions in ABR thresholds may represent replacement of hair cells or repair of the organ of Corti [17]. Improved CN VIII peak 5 latencies may represent repair of the spiral ganglion.
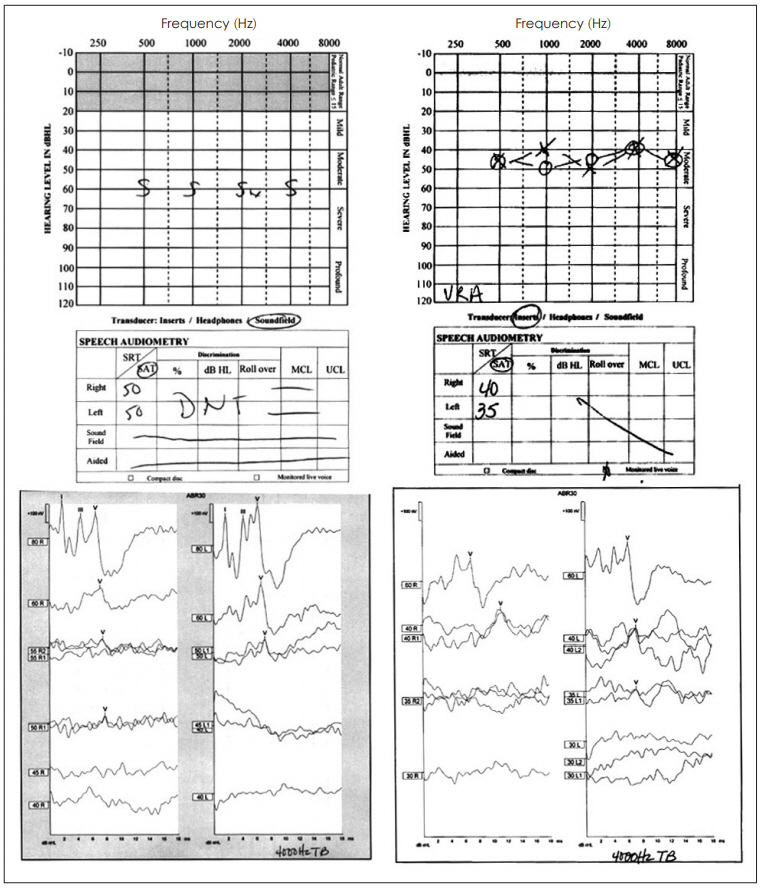
Representative audiograms (top) and ABR recordings at 4,000 Hz (below) of subject 5 before (left) and after (right) hUCB treatment. The improvements on the behavioral testing (audiogram) match the changes found on the ABR recordings (physiologic). hUCB: human umbilical cord blood, ABR: auditory brainstem response.
Regardless of response to hUCB treatment, 10/11 subjects’ standard scores on the Preschool Language Scale 4th edition remained stable or improved (Table 9). One study participant (Subject 7) dropped out of the trial before 6- or 12-month follow-up testing could be completed. Subject 4, who had poor compliance with recommended hearing aid use and speech therapy, experienced a decline in standard language scores despite an improvement in ABR threshold measures. Subject 4 reinforces the necessity of adequate amplification and therapy for children with SNHL.
Statistical results
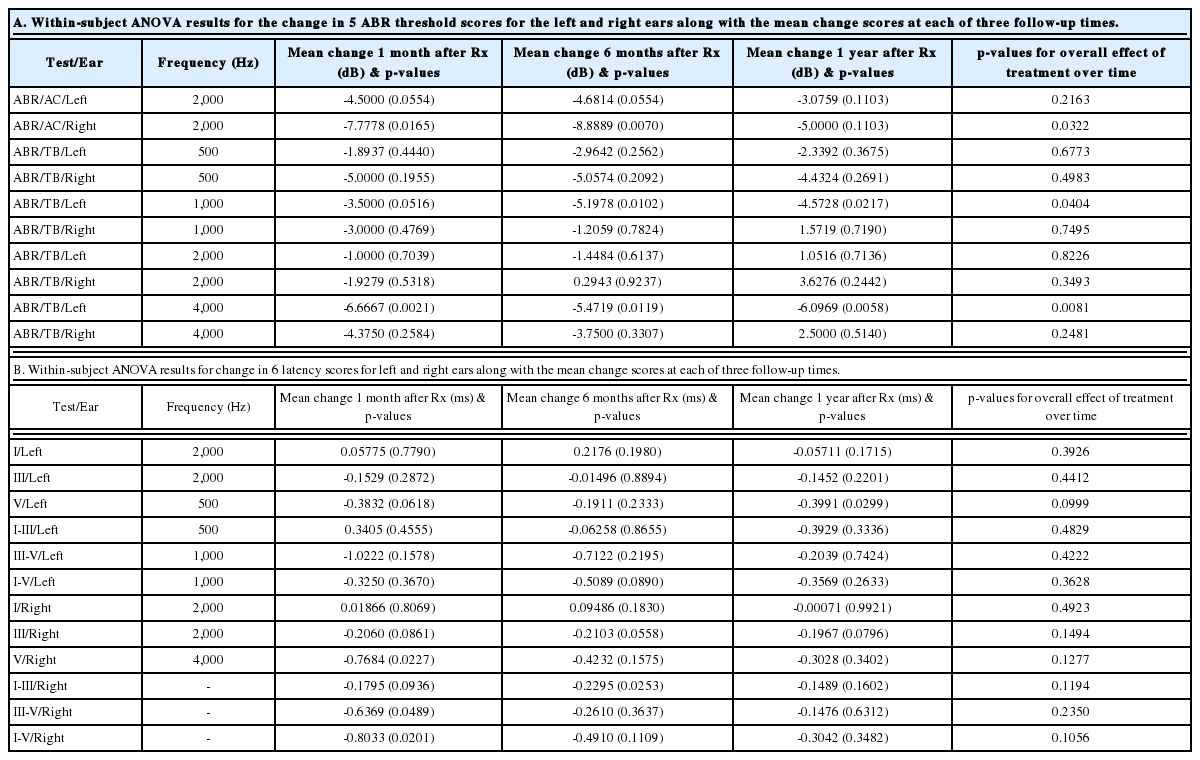
The overall effect of treatment is statistically significant (p<0.05) for ABR click right, TB left at 1,000 Hz and TB left at 4,000 Hz (Table 8A). Although the change in threshold is statistically significant only for 3 of the 10 measurements, it is somewhat expected due to the very small sample size and some missing data within the small pool. However, among 30 possible mean change scores at the 3 follow-ups, 25 showed improvement (Table 8A). Under the null hypothesis of no treatment effect, the probability of observing at least 25 improvements out of 30 is calculated using a binomial distribution to be only 0.00016. This is a very strong indication of the efficacy of the intervention that is not entirely captured by the individual significance tests for the threshold change scores.
The overall effect of treatment for latencies is not statistically significant for any of the measurements (Table 8B). However, among 36 possible mean change scores at the three follow-ups, 31 showed improvement (Table 8B). Under the null hypothesis of no treatment effect, the probability of observing at least 31 improvements out of 36 is calculated using a binomial distribution to be only 0.000006. This is a very strong indication of the efficacy of the intervention that is not reflected by the individual significance tests for the latency change scores.
MRI with DTI outcomes
FA is a sensitive marker of white matter integrity and myelination [18]. Decreased FA has been demonstrated in patients with SNHL [19,20]. No 12-month imaging data were available for 3 patients: subject 7 dropped out after the 1-month follow-up, subject 5’s parents refused 12-month imaging, and subject 11 underwent bilateral cochlear implantation before his 6-month follow-up. FA changes were found in 3 of the 5 subjects with improved ABR thresholds (subject 5’s parents declined 12-month follow-up imaging and subject 6 showed no change in anisotropy). Subject 1, who did not improve on the audiologic measure, showed a positive change in anisotropy within Heschl’s gyrus, although this was less robust than the changes found in the other subjects. The mean FA comparing subjects with ABR threshold improvement (responders) to subjects without ABR threshold improvement (non-responders) at ROI sites within Heschl’s gyrus are shown in Fig. 3A. Maximal FA at ROI sites along the auditory pathways are shown in Fig. 3B.
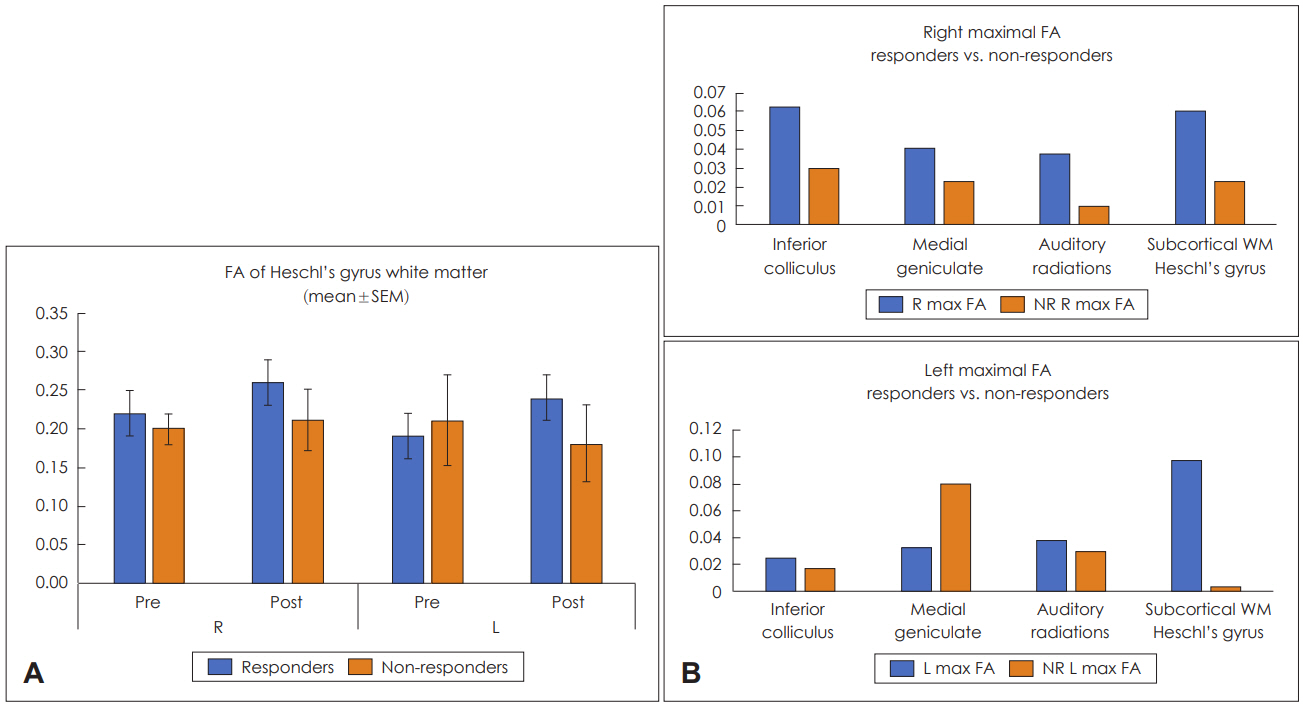
A: Graphic representation of mean FA in the region of interest of Heschl’s gyrus white matter comparing responding and non-responding study subjects before (pre) and after (post) hUCB treatment. B: Graphic representation of maximal FA at selected sites along the auditory pathways comparing responding (R) and non-responding (NR) study subjects following hUCB treatment. FA: fractional anisotropy, hUCB: human umbilical cord blood, SEM: standard error of the mean.
Our study demonstrates increased mean FA primarily within the white matter of Heschl’s gyrus in some patients who experienced an improvement in audiologic function following hUCB infusion, although this was not statistically significant. The data suggest that hUCB treatment can allow progressive myelination and strengthened integrity of auditory pathways in children with acquired SNHL.
Discussion
Our data demonstrate that the infusion of autologous hUCB to children with SNHL is safe. There was no evidence of infusion-related toxicity in pulmonary, hepatic, hematologic, renal or neurological organ systems. Further, autologous hUCB is feasible within the context of a children’s hospital with bone marrow transplant expertise. Improvement in ABR threshold, when observed, was evident on testing at one-month post treatment and durable over the 12-month follow-up period.
Our phase 1 study evaluating the potential toxicity of autologous hUCB infusion in children with SNHL followed an uncontrolled design. Because the cochlea is post-mitotic at birth and because spontaneous repair of the cochlea has not been reported, we compared pre-treatment cochlear function to post-treatment cochlear function. Although improved structural, behavioral and functional outcomes were observed, our study is underpowered and not designed to conclude any difference with treatment. However, the observed safety of the protocol and promising pre-clinical research showing a benefit from hUCB-derived cell therapy for SNHL warrants the implementation of controlled phase 2 trials.
Rationale for cell type, dosing and route
Two major classes of progenitor cell-based therapies are commonly utilized: autologous and allogenic. We chose to use autologous hUCB for many reasons: 1) no immune barrier considerations, 2) no in vitro culture/scaling issues for autologous applications, 3) ready availability, 4) no concerns regarding uncontrolled replication as with embryonic or fetal cells, and 5) no ethical objections to cell type.
As ours is the first trial to prospectively evaluate the use of autologous hUCB infusion for acquired SNHL, appropriate cell dosing was not defined at the beginning of our study. The cell dose was increased as the study proceeded. We saw no infusion-related toxicities but did note improvement in ABR thresholds and 8th cranial nerve peak V latencies in some subjects receiving greater than 15×106 TNCs/kg. Behavioral testing results (audiograms) correlated with physiologic (ABR) improvement (Fig. 2). These results provide direction for the establishment of a dose-response curve in subsequent trials.
We chose to deliver our hUCB intravenously because of the well-established safety of the administration of this cell type using this route in children with hematologic disease. In addition, a treatment effect was observed in pre-clinical trials using the intravenous delivery of hUCB-derived progenitor cell preparations. Further, the risks and potential complications of a direct surgical delivery of progenitor cells to the cochlea were avoided.
Functional outcome
Most (5/8) subjects receiving greater than the threshold dose of 15×106 cells/kg experienced a durable reduction in their ABR thresholds following hUCB treatment (Table 3). When the pre-treatment ABRs were used as a control measure, this improvement was statistically significant for the entire study population at several frequencies (Table 8A). In addition, cranial nerve 8 wave 5 latencies improved in 4/6 subjects treated above threshold (Table 4-7). For properly amplified subjects receiving appropriate speech-language therapy, language development was normal following treatment (Table 9).
Structural correlates to functional outcome data
Our study included high-resolution MRI imaging with DTI sequences before and one year after hUCB treatment. We included an analysis of FA at sites along the auditory pathways. FA is a measure of white matter tract integrity, and increased FA suggests white matter tract repair. When FA measures were compared between responding and nonresponding subjects (Fig. 3), a trend suggesting increased FA along these pathways in responding subjects was identified. The improvement in FA was most pronounced in the primary auditory cortex (Heschl’s gyrus). Taken together, our phase 1 data suggest that repair of the cochlea (ABR), spiral ganglion and the entire auditory pathway may be possible after hUCB treatment.
Potential mechanisms of action
Cochlear hair cell regeneration could be caused by direct interaction with hUCB cells, as well as by local or systemic paracrine effects caused by hUCB infusion. The cochlea is known to have resident macrophages, and macrophages can be recruited to the cochlea from circulating monocytes to damaged and dying inner hair cells [21,22]. A subset of cochlear support cells have been characterized as “stem-like” progenitor cells [23]. Recent pre-clinical studies have demonstrated the migration of human mesenchymal stem cells to the cochlea of congenitally deaf albino pigs and immunocompromised mice deafened by kanamycin [24,25]. In the mouse model, the mesenchymal cells were found to have fused with cochlear support cells, and hair cell regeneration was felt to be secondary to a local paracrine effect. In the pig model, umbilical cord mesenchymal stem cells were identified within the cochlea, and the treated animals’ ABRs showed improvement.
Following intravenous infusion, the majority of hUCB cells do not cross the blood-brain barrier [10,26]. Intravenous delivery of mesenchymal stem cells is known to alter circulating cytokines and macrophage cell phenotype. While it is possible that some hUCB cells may reach the cochlea and directly induce repair [24], it is also possible that this immune modifying treatment may allow the differentiation of resident cochlear cells into new hair cells through a systemic paracrine effect [27]. Cochlear progenitor cells may be induced to differentiate into hair cells by local or systemic paracrine effects, direct contact with hUCB cells, or a combination of these processes. Repair of the spiral ganglion and the cells contributing to the auditory pathways through similar mechanisms is also possible [8-14].
Treatments to induce hearing recovery using gene therapy or the direct delivery of stem cells, viruses, or small molecules directly into the cochlea are currently under investigation [27-30]. In addition to the potential surgical morbidity, these approaches focus narrowly on cochlear repair and do not address the associated pathways necessary for hearing and language. These direct delivery approaches might be enhanced by hUCB treatment, which appears to target the cochlea, the spiral ganglion and the associated pathways for hearing.
In conclusion, our phase 1 open label study fulfilled the objective of evaluating the safety of intravenous autologous hUCB mononuclear fraction infusion for the treatment of acquired SNHL. We exceeded the minimal 6×106 TNC/kg in all patients, and the per kilo cell dose was increased throughout the trial. Functional and associated structural improvements were observed in several subjects receiving higher perkilogram doses of cord blood cells. The range in age of the responding subjects at treatment (9 months to 3 years 7 months) suggests a long window of opportunity for effective treatment. Because of the limited sample size, our study is underpowered and not designed to make conclusions on differences due to treatment. ABR thresholds would not be expected to spontaneously improve in this patient population. The observed safety of the protocol and promising clinical data suggest a benefit from cord blood-derived cell therapy for acquired SNHL. The data warrant the implementation of larger controlled phase 2/3 trials of this intervention in children with acquired SNHL.
Acknowledgements
This study was supported by a grant from Cord Blood Registry® (CBR®). Additional support was provided by Wayne Densch Charities.
Notes
Conflicts of interest: The authors have no financial conflicts of interest.