Speech Perception and Gap Detection Performance of Single-Sided Deafness under Noisy Conditions
Article information
Abstract
Background and Objectives
Many studies have reported no benefit of sound localization, but improved speech understanding in noise after treating patients with single-sided deafness (SSD). Furthermore, their performances provided a large individual difference. The present study aimed to measure the ability of speech perception and gap detection in noise for the SSD patients to better understand their hearing nature.
Subjects and Methods
Nine SSD patients with different onset and period of hearing deprivation and 20 young adults with normal hearing and simulated conductive hearing loss as the control groups conducted speech perception in noise (SPIN) and Gap-In-Noise (GIN) tests. The SPIN test asked how many presented sentences were understood at the +5 and -5 dB signal-to-noise ratio. The GIN test was asked to find the shortest gap in white noise with different lengths in the gap.
Results
Compared to the groups with normal hearing and simulated instant hearing loss, the SSD group showed much poor performance in both SPIN and GIN tests while supporting central auditory plasticity of the SSD patients. Rather than a longer period of deafness, the large individual variance indicated that the congenital SSD patients showed better performance than the acquired SSD patients in two measurements.
Conclusions
The results suggested that comprehensive assessments should be implemented before any treatment of the SSD patient considering their onset time and etiology, although these findings need to be generalized with a large sample size.
Introduction
Single-sided deafness (SSD) refers to a condition in which a patient has non-functional hearing on one side (higher thresholds than 70 dB HL) and normal hearing on the contralateral side (lower threshold than 25 dB HL) [1]. Due to using only one ear in daily life, SSD patients experience multiple audiological difficulties, such as challenges understanding speech, especially in noisy backgrounds, and detecting sound sources from localization [2-4], resulting in deterioration of their hearing performance compared to that of normal hearing listeners with binaural cues [5].
Contemporary researchers have introduced the bone-anchored hearing aid (BAHA) and its benefits as one treatment option for SSD patients. After BAHA implantation, many patients showed lower (or better) speech perception thresholds under noisy circumstances [6-8] and reported receiving a significant number of benefits while providing decreased values in the hearing handicap index [6,8]. Regardless, they still had hearing troubles in the source localization [7]. In short, such findings did not indicate direct improvements to the peripheral auditory system, such as pinna and cochlea located in the implanted side, although previous studies did not show longitudinal and consistent results. Rather, implantation might lead to a partial gain in the central auditory system.
Based on Kral, et al.’s [9] animal study in 2013, a considerable difference in brain reorganization occurs between binaural deafness and the SSD in terms of a critical period for development. In the human auditory system, most cortical synapses have completed development during the first 1-2 years [10]; thus, the cortical developmental sequence of infants with binaural deafness could be severely affected by a total lack of auditory experience. Meanwhile, SSD patients have unique cortical development due to highly imbalanced input from the two ears, depending on onset and/or duration of the deprivation in one deaf ear; consequently, their central auditory system is reorganized in that some synapses not activated by the deaf ear could ultimately be replaced by synaptic contacts from the active healthy ear [9]. Here, we might suspect that just a long period of the hearing deprivation is not related to the poor function of the central auditory system for SSD patients. In the same vein, the central auditory performance of SSD needs to be evaluated, and their outcomes should be scrutinized, which might provide a specific preoperative clinical guideline of BAHA candidacy in terms of central auditory benefits. Nevertheless, none of the previous studies have conducted typical tests of central auditory function for SSD patients. For example, SSD patients’ speech discrimination ability was measured by the hearing threshold in noise (HINT) test in previous studies [6-8], but we have to differentiate it from speech perceptual/understanding scores in the background noise; the former aims to find the threshold to hear speech with a 50% chance whereas the latter determines how well the patient understands the sentence under environmental noise, such as the speech perception in noise (SPIN) test. In other words, the HINT scores failed to represent a performance of frequency resolution, and thus many studies misreported significant changes in hearing thresholds as an improved speech perception ability. It might lead to incomprehensible results as improved speech perception in noise (not real perceptual changes) from no improved sound localization.
To understand that human speech consists of various complex factors, at least a simple approach to the frequency and timing characteristics is needed. For the temporal processing ability, which is an important aspect in central auditory processing [11], Glasberg and colleagues measured gap thresholds for unilateral hearing-impaired subjects [12]. They concluded that impaired subjects had a large gap threshold, which increased as the hearing threshold increased; however, the data presented a large scatter in their gap thresholds. The need to study the large variance carefully warranted the current study. Appropriate information regarding the frequency and temporal resolutions for SSD patients could provide a better understanding of their characteristics [5]. Thus, the clinicians expect concrete benefits of audiological treatments. Considering this, the current study’s research questions deal with individual factors in the existing large variance affecting the speech recognition performance and temporal processing ability. The first hypothesis is that, as the level of noise increases, the performance of speech recognition could decrease in SSD patients. The second hypothesis is that SSD patients show poorer performance in both speech perception and gap detection under background noise than listeners with normal hearing and/or simulated temporary hearing loss listeners not affected by brain plasticity. The final hypothesis is that there is a distinguishable difference in our experimental results depending on the time of SSD onset or onset age, similar to Kral, et al.’s [9] animal model.
Subjects and Methods
Participants
Between April and August in 2018, we recruited patient volunteers who visited the Wonju Severance Christian Hospital and had typical audiological criteria indicating single-side deafness. The exclusion criteria were as follows: 1) thresholds lower than 25 dB HL in the good ear and higher than 70 dB HL in the poor ear, 2) any acute or sudden hearing loss under medical treatment, and/or 3) chronic hearing loss for less than 1 year. After the criteria were confirmed, nine patients with SSD (referred to as the SSD group) were recruited for the study. They ranged in age from 20 to 81 years [mean (M)= 57.22, standard deviation (SD)=23.86] and consisted of six males and three females. In the SSD group, their etiology was chronic otitis media, vestibular schwannoma excision, labyrinthitis, sudden sensorineural hearing loss, noise-induced hearing loss, and congenital deafness. Their period of hearing loss varied from 2 years to more than 50 years. Table 1 lists each participant in the SSD group.
As counterparts to the SSD group, 20 young adults with normal hearing [i.e., the normal hearing listener (NHL) group] were recruited for participation. They reported a negative history of head and neck abnormalities, ear surgery, otologic disease, and head trauma. They conducted hearing screening and showed a normal range of hearing thresholds (≤15 dB HL) in the test frequency between 125 Hz and 8,000 Hz in the pure-tone audiometry and A-type of tympanogram to ensure normal middle ear function. Two weeks after the first test day, the NHL group instantly developed unilateral conductive hearing loss [referred to as the unilateral hearing loss (UHL) group] by using impression material in their right ear equally. Impression material used medium viscosity silicone which regarded appropriate material to block the ear canal. The average amount of hearing loss for UHL group was 42.33 dB HL (SD: 5.41 dB).
All participants in all three groups were native Korean speakers and completed an informed consent form before participating in the experiment. The experimental procedures were approved by the Institutional Review Board of Wonju Severance Christian Hospital (IRB 19-003).
Test materials
For the speech perception performance, the Korean Speech Perception in Noise (KSPIN) test was used [13]. After removing a question tag from the original version in order to minimize a possible extra hint in the sentence [14], the sentences were adjusted by an equal root mean square of -20 dB. Twelve lists having 20 sentences were randomized within the groups and the lists had an equal level of difficulty [15]. In the task, two signal-to-noise ratio (SNR) conditions (i.e., +5 and -5 dB) were applied with 12 multi-talkers’ babble noise, which functioned as informational masking [16]. After listening to the sentences presented via a compact disk (CD) player, the participants were asked to repeat back what they heard.
To identify the temporal processing, a Gap-In-Noise (GIN) test was conducted, again using the CD player [11]. In the GIN test, the duration of gaps randomly consisted of 10 series lasting from 2 to 20 ms. The participants were asked to press a button whenever they heard gaps of different lengths within the continuous white noise.
Experimental procedures
During both KSPIN and GIN experiments, the participants were seated at 1 meter and 45 degrees azimuth from the loud speaker in a sound isolation booth. The CD player was connected to an audiometer (Model GSI 61; Grason-Stadler, Eden Prairie, MN, USA), and the presentation level of the two tests was bilaterally set at the most comfortable level (MCL) to each subject. Although all groups’ better ear was less than 25 dB HL in threshold, the MCL of three groups was 51.25, 54.78, and 69.44 dB HL for the NHL, UHL, and SSD groups respectively, since their poor ears were different.
The test conditions of both KSPIN and GIN were pseudo-randomized across the participants; half did KSPIN first and the others did GIN, and the two levels of background noise were also randomized. Before testing the GIN thresholds, a practice session of 5 trials was provided to become familiar with detecting the gap [11]. After completing the tests, the error percentage of the sentence list and the shortest gap in the noise were designated as the results.
Data and statistical analysis
In the KSPIN test, sentence error percent was calculated while including missing words, synonyms, and grammatical errors. The results of the GIN test were analyzed as the threshold of the shortest gap that might be detected more than four times among the six times total per gap while following the original protocol [11]. For a comparison of the group mean, a statistical analysis was conducted using SPSS software (ver. 22, IBM Corp., Armonk, NY, USA). A one-way analysis of variance (ANOVA) with repeated measures was conducted to identify the main and interaction effects of SPIN and GIN test results. If necessary, a Bonferroni post hoc test was applied with multiple comparisons.
Results
Speech perception in noise
As demonstrated in Fig. 1, the NHL and UHL groups showed a much lower error percentage in the KSPIN test at both +5 and -5 dB SNR. There was a significant main effect in speech perception among these groups for the +5 dB noise condition [F(2, 45)=6.206, p<0.01] and the -5 dB noise condition [F(2, 45)=16.295, p<0.01]. Under the +5 dB condition, the NHL group showed the lowest error percentage (M: 11.25%, SD: 11.34) and the UHL group (M: 12.00%, SD: 9.65) showed a significantly lower error percentage than the SSD group (M: 28.33%, SD: 26.46). In the -5 dB condition, the NHL group (M: 18.75%, SD: 11.22) showed the lowest error percentage among the three groups. The UHL group (M: 20.25%, SD: 11.64) showed a higher error percentage than the NHL group but was not statistically significant. As we expected, the SSD group showed a higher error percentage (M: 54.44%, SD: 29.20) than both the NHL and UHL groups. Interaction was also found between these groups and noise levels as the SSD group had an outstanding pattern of abrupt increasing error percentage from +5 to -5 dB SNR.

A mean comparison of sentence error among the three experimental groups at +5 dB SNR (A) and -5 dB SNR (B). Dots refer to outliers. NHL: normal hearing listener, UHL: unilateral hearing loss, SSD: single-sided deafness.
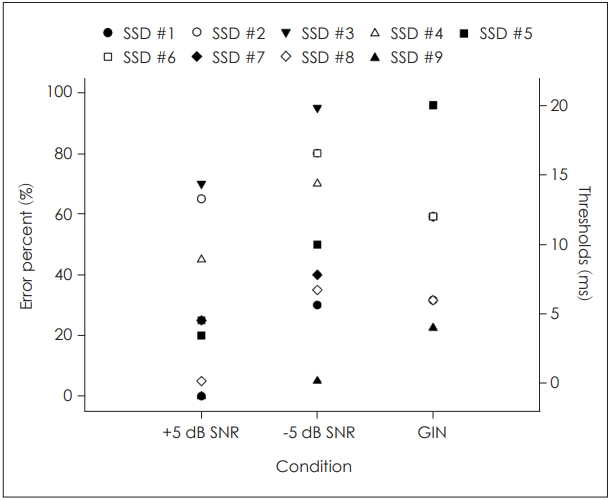
When looking at our data more closely, the SSD patients had much larger distribution within the group (i.e., 0% to 75% error rate) than the other groups, and its distribution was increased from a 5% to 95% error rate at the higher background noise level (Fig. 2). For example, SSD #8 and #9 in Table 1, who were cogenital SSD for more than 20 years, had the lowest errors within the group. However, although SSD #7, who had congenital one-ear deafness but was much older than SSD #8 and #9, showed not such a good performance, which was possibly due to the aging effect in the central auditory region. On the other hand, the acquired SSD patients showed a higher error percentage, except for SSD #1 with chronic otitis media.
Gap-In-Noise
Fig. 3 showed the mean comparison of the temporal resolution for three experimental groups. A significant main effect of the GIN test [F(2, 45)=21.312, p<0.01] was confirmed. In the GIN test, the NHL group (M: 4.59 ms, SD: 0.68) showed a better threshold than the UHL group (M: 5.35 ms, SD: 1.14), but was not significant. The SSD group (M: 10.00 ms, SD: 5.00) showed significantly poor thresholds and the largest variance in all groups. The results of the Bonferroni correction indicated that the SSD group had longer thresholds than either the NHL or UHL groups (p<0.01).

A mean comparison of Gap-In-Noise thresholds among the three experimental groups. Dots refer to outliers. NHL: normal hearing listener, UHL: unilateral hearing loss, SSD: single-sided deafness.
Similar to the SPIN results, the nine SSD patients had a large distribution within the group compared to the other groups. As Fig. 2 and Table 1 demonstrate, SSD #7-#9, who had congenital SSD, and SSD #1, with chronic otitis media, had short gap thresholds of 4 to 6 ms in common. However, SSD #2-#6 who had acquired SSD showed rather longer GIN thresholds, although different etiology.
Discussion
The present study measured speech perception in background noise and GIN threshold in nine SSD patients while comparing their control groups of 20 listeners with normal hearing and 20 simulated instant unilateral hearing loss to confirm central auditory performance.
According to the first research hypothesis, we expected the error percentage of speech recognition performance to increase as the level of background noise increased in all groups, but the UHL groups showed a similar performance to the NHL group due to little effect on the central plasticity. The results from the performance of speech recognition ability under noisy environments were much poorer in the SSD group than in the NHL and/or UHL groups, which not surprisingly supported the results of many previous studies [2-5]. In the case of the -5 dB condition for speech recognition performance, as the level of background noise increased, the error percentages of sentence recognition increased in all groups. Moreover, the SSD group showed a higher error percentage and a larger variation than the NHL and UHL groups. This result could explain how the SSD group could be affected by binaural summation, binaural squelch effect, and head shadow effect due to monaural hearing [17-19]. On average, all three groups-NHL, UHL, and SSD-in the -5 dB condition showed an increased error percentage in the speech recognition performance compared to the +5 dB condition. In particular, the SSD group showed a significantly greater error percentage and variance than the other groups while supporting our second hypothesis. This result showed similar results as Firszt, et al. [20] who conducted cochlear implants on patients with asymmetric hearing loss. They found that the correct percentage of speech recognition was higher in conditions quieter than -2 dB SNRs. However, the results of speech recognition performance demonstrated obvious differences in both SPIN and HINT in terms of purpose, stimuli, and ways to response the stimuli, but the performance of speech recognition decreased as the level of background noise and hearing thresholds increased.
Why did the SSD patients have such a larger individual difference? When looking at the individual data, we found that the SSD patients might have brain plasticity due to having monaural hearing for a longer period [9]. In other words, congenital and acquired SSD patients differed in this critical period. Kral, et al.’s study [9] showed that a sensitive period for cortical aural reorganization after unilateral deafness in animal models indicated a reduced number of central projections from the cochlear nucleus of the side ipsilateral to the ablated cochlea. Po-Hung Li, et al. [21] further supported this finding with data from nine humans with SSD in that a strong reorganization of aural input to the cochlear nucleus was shown as early as 3 weeks after the onset of hearing loss. Therefore, cortical auditory response of SSD patients might be firmed up in a very early period. Many clinicians have reported that most SSD patients had decreased (or better) threshold in hearing noise after BAHA or cochlear implant (CI) implantations [1,6,8]; however, frankly speaking, their perceptual performance was not changed. Consequently, it should be classified as their onset of SSD by either congenital or acquired factors [2].
In terms of the temporal processing ability measurement, the GIN test showed a comparative data of gap detection among our three groups and it also supported their KSPIN results with the similar pattern. In detail, although the NHL and UHL showed a relatively normal range of GIN test thresholds compared to the normative data (i.e., 4.8 ms and 4.9 ms for each left and right ear in young adults, respectively) for previous study [11], the SSD group demonstrated poorer thresholds than other groups. As we expected, the SSD group showed poorer hearing thresholds and gap detection thresholds, which relatively decreased the temporal processing ability, than the NHL and UHL groups. This result partially supported the findings of previous studies for the temporal resolution [11,12,22]. The SSD group may be affected by the hearing loss and long duration of hearing deprivation, and they showed a poorer temporal processing ability than the UHL group, which was not affected by the central portion [9]. Central plasticity stems from the duration of hearing deprivation, and decreased hearing thresholds might have affected the GIN test results [11,12]. Again, the UHL was subjected to simulated temporary conductive hearing loss, which led to a decrease in the peripheral auditory ability, such as hearing thresholds, not the central one. Surprisingly, a wide variance of gap thresholds from 4 ms to 20 ms in the SSD patients matched the results from the frequency resolution. In other word, SSD patients with a large error in the KSPIN test also had a longer GIN threshold and vice versa, while supporting our third hypothesis that there existed in a distinguishable difference depending on the time of SSD onset or onset age (i.e., congenital vs. acquired SSD). Although it does not explain well the reason why such difference may determine central auditory performance for our SSD patients, we are better to revisit the study of Kral, et al. [9] and these results are applicable for treatment of SSD patients as the important factor.
The present study includes four limitations that could be helpful to further ongoing studies. One of the limitations was the inconsistencies for SSD patients. Large variances emerged in the SSD patients’ clinical background, such as etiology and duration of hearing deprivation, even though it was our intention to find specific reasons from such variances in the study design. This could be working for another effect of the wide range of variances in audiological performance. For a possible clinical application of the current results, it is important to have consistent clinical backgrounds among SSD patients. To figure out SSD patients’ individual factors, clustering the groups and analyzing the affecting factors are essential. These efforts may lead to identifying the individual factors affecting the auditory performances as well as the possible effect of changes in central and/or auditory plasticity. Second, the wide range of ages in the SSD groups stemmed from another limitation of the current study. Although the abilities of speech perception with noise and temporal processing could be affected by aging, the present study could not consider the possible effects of aging. SSD #1, 2, 3, and 6 who are older than 65 years old had relatively high performance for the KSPIN result under +5 dB SNR compared to SSD #8 and 9 who are young adults. In addition, their results of -5 dB SNR condition were more deteriorated than that of +5 dB SNR condition. Similar to the KSPIN test, the results of GIN test showed deteriorated thresholds in the older adults, except for SSD #1 and 7 patients. Third, either KSPIN or GIN test is not representative measurement of the central auditory function but is a simple clinical measurement. Thus, considering current results of the study, we extend to evaluate various hearing natures of SSD patient to better understand and threat them. Fourth, the thresholds of each SSD and UHL group were not consistent. Although the present study tried to compare SSD patients with UHL group which designated to instantly conductive hearing loss to control the brain plasticity not the peripheral one, the differences in the hearing thresholds could be affect the results of both speech perception and gap detection performance. Moreover, while the type of simulated hearing loss of present study was conductive hearing loss which based on the second hypothesis, it is necessary to figure out the differences between simulated conductive or sensorineural hearing loss. Finally, although the sample size of the present study calculated power analysis using G*Power [23] assuming an α level of 5%, the statistical output could be less powerful due to each experimental group’s small size. Thus, the current results should be generalized with a large sample size in subsequent studies. The results could then be used to predict audiological performance after the implantation of hearing-assistive devices [e.g., hearing aid (HA), CI, and BAHA].
The results of current study implied that both KSPIN and GIN test could be useful tool for SSD patients to assess their frequency and temporal resolutions. Although SSD patients showed various audiological difficulties (i.e., hard to understand speech signal in challenging environments and to localize sound sources), the hearing-assistive devices such as HA, CA, and BAHA could be helpful tool to compensate the difficulties stems from the monaural hearing. Especially for the BAHA, many of previous studies reported that the BAHA recipients had decreased (or better) speech perception thresholds in the presence of background [6-8] and score for self-report questionnaire which assess the hearing handicap index [6,8]. The performance for both before and after BAHA implantation may varied by individually. In terms of selection and prediction for the performance of BAHA recipients, it is important and meaningful to confirm the frequency and temporal resolution ability.
Acknowledgements
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2018S1A3A2074932).
Notes
Conflicts of interest: The authors have no financial conflicts of interest.
Author Contributions: Conceptualization: Chanbeom Kwak and Woojae Han. Data curation: Chanbeom Kwak, Saea Kim, and Jihyeon Lee. Formal analysis: Chanbeom Kwak. Project administration: Youngjoon Seo and Woojae Han. Validation: Taehoon Kong. Writing—original draft: Chanbeom Kwak. Writing—review and editing: Woojae Han.