Clinical Factors Influencing the Trial and Purchase of Bilateral Microphones with Contralateral Routing of Signal in Patients with Asymmetric Sensorineural Hearing Loss
Article information
Abstract
Background and Objectives
Bilateral microphones with contralateral routing of signal (BiCROS) hearing aid is an option for hearing rehabilitation in individuals with asymmetric sensorineural hearing loss (ASNHL). The clinical factors influencing the trial and purchase of BiCROS were investigated.
Subjects and Methods
We reviewed the medical records of 78 patients with ASNHL who were recommended to use BiCROS and analyzed the demographic and audiological factors influencing the trial and purchase of BiCROS.
Results
Among the 78 patients, 52 (66.7%) availed of the free BiCROS trial and 21 (26.9%) purchased BiCROS. The mean pure tone audiometry (PTA) air conduction (AC) threshold of the better- and worse-hearing ears were 44.2±12.8 dB and 90.7±22.5 dB HL, respectively. The decision for trial or purchase of BiCROS was not influenced by age, sex, duration of hearing loss of the worse-hearing ear, or PTA AC threshold or speech discrimination score of both ears. The first and third quartiles of the PTA AC thresholds for the better-hearing ear of BiCROS buyers were 38.75 dB and 53.75 dB HL, respectively. The counterpart values for the worse-hearing ear were 72.50 dB and 118.75 dB HL, respectively.
Conclusions
The clinical factors analyzed in this study were found to be irrelevant to the trial and purchase of BiCROS in patients with ASNHL. Nevertheless, the distribution range of the auditory thresholds of the subjects using BiCROS can be a useful basis for the counseling of patients with ASNHL and selection of candidates for BiCROS use.
Introduction
Individuals with asymmetric sensorineural hearing loss (ASNHL) report discomfort in daily living, both audiologically and psychosocially [1,2]. Functional impairment in monaural hearing results not only from decreased loudness but also from poor localization of sound and poor speech recognition in noise [3]. Individuals with single-sided deafness lack the ability to localize sounds in the horizontal plane based on the interaural time difference and interaural level difference [4]. These hearing difficulties consequently affect an individual’s social and psychological well-being.
There are several options for the hearing rehabilitation of ASNHL [5]. One strategy is cochlear implantation, a surgical method currently and commonly employed, which enables hearing through the worse-hearing ear [6]. Another strategy is to transfer sounds coming from the worse-hearing ear towards the better-hearing ear, thus overcoming the head shadow effect. This transfer of sound is possible through bone conduction (BC) systems and air conduction (AC) systems [7-11]. Transcranial BC of sound is achieved through surgically implanted bone-anchored hearing aid (BAHA) [12] or nonsurgical BC systems, such as BAHA Softband (Cochlear, Australia) or ADHEAR system (MED-EL, Austria). Conversely, rerouting of signals from the worse- to the better-hearing ear through AC of sound is the principle behind the contralateral routing of signal (CROS) and bilateral microphones with CROS (BiCROS) systems [13,14].
CROS/BiCROS AC hearing aids utilize a transmitter unit with a microphone and a receiver unit with or without an amplification function. A transmitter in the worse-hearing ear delivers sound to the receiver in the better-hearing ear transcranially via a wire or wirelessly [15-17]. BiCROS hearing aids can amplify sounds both from the transmitter side and the receiver side. Therefore, CROS can be recommended to patients with unilateral sensorineural hearing loss with normal hearing in the better ear but unaidable hearing in the worse ear; conversely, BiCROS is more suitable for patients with ASNHL with both ears have hearing loss but the better ear having substantially better hearing than the other.
However, the CROS/BiCROS systems still have limitations. The rerouting of sound can overcome the head shadow effect, thereby improving the ability to understand speech in background noise. However, this can also minimize the difference in acoustic signature towards both ears, which can interfere with the access to monaural level cues needed for sound localization in the horizontal plane [18,19]. The limited acceptance of CROS/BiCROS devices despite the known practical advantages can be attributed to the difficulty in appropriate candidate selection and poor performance factors, such as ineffectiveness in noisy conditions [20]. Therefore, BiCROS candidates should be selected after proper consultation based on individual audiometric profiles and other clinical considerations. Currently, there is no established consensus on the suggested audiometric configuration for BiCROS use.
The consultation process for BiCROS use usually includes an introduction of the device, a trial for several weeks, and then guidance to the purchase of the BiCROS device according to the patient satisfaction. Some authors have previously reported the factors influencing the decision for BAHA use [21]; however, studies regarding the consultation and decision for BiCROS use are still lacking.
The present study aimed to analyze the clinical factors influencing the trial and purchase of BiCROS and to propose an ideal audiometric profile of BiCROS users based on individual clinical data. In addition, the authors assessed the consultation process for BiCROS use and obtained numerical data with respect to the decision for satisfactory BiCROS use in patients with ASNHL.
Subjects and Methods
Subjects
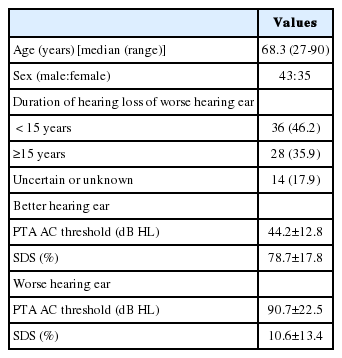
The data of 78 subjects (43 males and 35 females, age range: 27-90 years, mean age: 68.3 years) with ASNHL were reviewed retrospectively. The patients visited the otology outpatient clinic of a tertiary care center (Seoul National University Bundang Hospital) between January 2016 and October 2018. Patients with active chronic suppurative otitis media or history of open-cavity tympanomastoidectomy were excluded. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital, Republic of Korea (IRB No. E6-2016-1106).
Patient evaluation and BiCROS use consultation
All patients were evaluated individually by a single clinician (BYC) based on their subjective complaints, pure tone audiometry (PTA) and speech audiometry (SA) results, clinical status, and socioeconomic status. PTA AC and BC thresholds were obtained at 0.25, 0.5, 1, 2, 3, and 4 kHz. The four-frequency average of the pure tone thresholds was calculated as the average threshold at 0.5, 1, 2, and 3 kHz. Speech recognition thresholds and speech discrimination scores (SDSs) were assessed using SA. ASNHL was defined as binaural differences in the PTA BC thresholds of >10 dB HL at two consecutive frequencies or >15 dB HL at one frequency (0.25-8.0 kHz).
After the counseling at the outpatient clinic room, the subjects were guided to the room for hearing rehabilitation for the introduction to BiCROS. Audeo B30 hearing aid (Phonak, Zurich, Switzerland; RIC type, eight channels) with noise reduction function was provided for the better-hearing ear, while CROS B-312 (Phonak, Zurich, Switzerland; RIC type) was suggested for the worse-hearing ear. The subjects were informed on how to manipulate, wear, and use the BiCROS. Thereafter, the subjects were allowed to experience a free 2- to 4-week trial of BiCROS for use in daily living. Following the trial session, the patients were followed up at the outpatient clinic and decided whether to purchase the BiCROS device. The same devices were suggested; however, some patients seeking for better performance purchased Audeo B50 (Phonak, Zurich, Switzerland; 12 channels), Audeo B70 (Phonak, Zurich, Switzerland; 16 channels), or Audeo B90 (Phonak, Zurich, Switzerland; 20 channels) for the better-hearing ear.
Statistical analysis
The results were analyzed using SPSS 18.0 (SPSS Inc., Chicago, IL, USA). Audiological parameters, such as AC thresholds in PTA or SDS in SA, were compared according to the BiCROS trial status or purchase status using the Mann-Whitney test. Demographic factors, such as age and sex, and the duration of hearing loss in the worse-hearing ear were assessed with respect to the BiCROS trial or purchase status using the Mann-Whitney test or chi-square test, depending on the variables. A univariate logistic regression analysis was performed to determine the factors influencing the decision to try or purchase BiCROS. p-values of less than 0.05 were regarded as statistically significant.
Results
General audiological characteristics
The audiometric assessment of the 78 patients revealed a wide degree of ASNHL. The duration of hearing loss of the worse-hearing ear was less than 15 years in 36 subjects (46.2%) and more than 15 years in 28 subjects (35.9%), while it is uncertain or unknown for the remaining 14 subjects (17.9%). The PTA AC threshold of the better- and worse-hearing ears were 44.2±12.8 dB and 90.7±22.5 dB HL, respectively. The SDS of the better- and worse-hearing ears were 78.7± 17.8% and 10.6±13.4%, respectively (Table 1).
Consultation process for BiCROS use
Among the 78 subjects with ASNHL, 52 patients (66.7%) elected to try a BiCROS device for 2 to 4 weeks. Twenty-one patients (26.9%) finally decided to purchase a BiCROS device for permanent use. Three of them purchased the device without a free trial period (Fig. 1).
Clinical factors influencing the trial of BiCROS
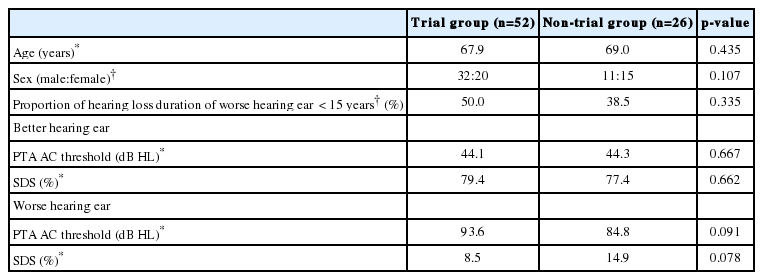
Initially, the clinical factors influencing the decision for BiCROS trial were assessed. The demographic factors (age and sex) and audiological parameters (duration of hearing loss of the worse-hearing ear, PTA AC threshold, and SDS of both ears) were compared between the trial group (n=52) and the non-trial group (n=26).
The mean patient age was 67.9 years in the trial group and 69.0 years in the non-trial group; however, there was no significant difference (p=0.435). There was also no significant difference in the male-to-female sex ratio between the trial group (32:20; male sex, 61.5%) and non-trial group (11:15; male sex, 42.3%) (p=0.107); however, the males tended to join the trial session more frequently than did the females. The proportion of hearing loss duration of less than 15 years was not different between the trial group (50.0%) and the nontrial group (38.5%) (p=0.335).
The PTA AC threshold and SDS of the better-hearing ear showed no difference between the trial (44.1 dB HL, 79.4%) and the non-trial (44.3 dB HL, 77.4%) groups (p=0.667 and p=0.662, respectively). The PTA AC threshold and SDS of the worse-hearing ear were not significantly different between the trial (93.6 dB HL, 8.5%) and the non-trial (84.8 dB HL, 14.9%) groups (p=0.091 and p=0.078, respectively); however, the patients with poorer function of the worse-hearing ear were more likely to join the BiCROS trial session (Table 2). The univariate logistic regression analysis for the trial of BiCROS revealed that the demographic and audiological factors were irrelevant to the decision.
Clinical factors influencing the purchase of BiCROS
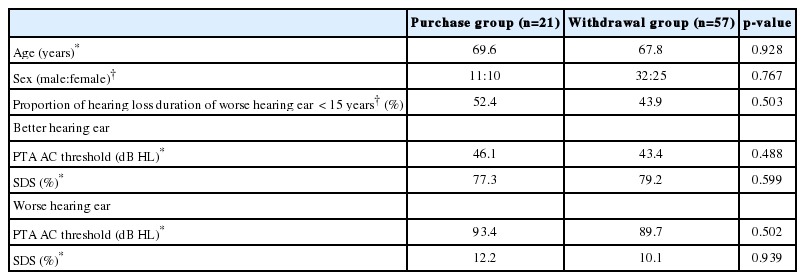
The clinical factors affecting the decision to purchase BiCROS were also analyzed in the same manner. The demographic and audiological factors were investigated for the purchase group (n=21) and the withdrawal group (n=57).
The average patient age in the purchase group (69.6 years) did not differ significantly from that in the withdrawal group (67.8 years) (p=0.928). The male-to-female sex ratio in the purchase group (11:10; male sex, 52.4%) showed no significant difference from that in the withdrawal group (32:25; male sex, 56.1%) (p=0.767). The proportion of hearing loss duration of less than 15 years was 52.4% in the purchase group and 43.9% in the withdrawal group, which was not significantly different (p=0.503).
The purchase group tended to have a lower hearing function of the better ear, although the PTA AC threshold and SDS of the better-hearing ear were not significantly different between the purchase group (46.1 dB, 77.3%) and the withdrawal group (43.4 dB, 79.2%) (p=0.488 and p=0.599, respectively). The PTA AC threshold and SDS of the worse-hearing ear were also not significantly different between the purchase group (93.4 dB, 12.2%) and the withdrawal group (89.7 dB, 10.1%) (p=0.502 and p=0.939, respectively) (Table 3). The univariate logistic regression analysis for the purchase of BiCROS revealed that the demographic and audiological factors were irrelevant to the decision.
Range of hearing function of the BiCROS buyers
The audiological parameters of the 21 individuals who purchased BiCROS were further investigated. The PTA AC thresholds of the better- and worse-hearing ears are shown in Fig. 2.

Pure tone audiometry air conduction (PTA AC) thresholds of the 21 BiCROS buyers. A: Better-hearing ear. B: Worse-hearing ear. C: Box plot of averaged PTA AC thresholds of both ears.
The median PTA AC threshold of the better-hearing ear was 45.00 dB HL, while the first and third quartiles were 38.75 dB and 53.75 dB HL, respectively. The median SDS of the better-hearing ear was 80%, while the first and third quartiles were 64% and 92%. Conversely, the median PTA AC threshold of the worse-hearing ear was 100.00 dB HL, while the first and third quartiles were 72.50 dB and 118.75 dB HL, respectively. The median SDS of the worse-hearing ear was 0%, while the first and third quartiles ranged from 0 to 32%.
Discussion
BiCROS AC hearing aids enable rerouting of signals from the worse- to the better-functioning ear. Patients with ASNHL are provided with an opportunity to experience the functional benefits of BiCROS during the free trial period and then decide whether to purchase the device. Among the 78 subjects with ASNHL who were counseled for BiCROS use in our study, 52 subjects (66.7%) availed the free trial session, and finally, 21 subjects (26.9%) elected to purchase the BiCROS device. The acceptance rates for CROS were previously reported as 10-20% for analog devices [20]; however, there are no recently published data regarding the rates for the trial or purchase of BiCROS in South Korea.
Our clinical analysis revealed that the patients with ASNHL who were counseled for BiCROS use showed a PTA AC threshold of 44.2±12.8 dB HL in the better-hearing ear and 90.7±22.5 dB HL in the worse-hearing ear. We found no evidence that age, sex, duration of hearing loss in the worse-hearing ear, or hearing function had any significant impact on the decision for the trial or purchase of BiCROS. This may be attributed to the fact that BiCROS was only suggested to selected patients with ASNHL based on the individual hearing function and need for hearing rehabilitation by an experienced otologist. Nevertheless, some notable tendencies could be found.
The male patients with ASNHL tended to comprise a larger proportion in the BiCROS trial group (61.5%) than in the non-trial group (42.3%), although this was not significant (p= 0.107). The male subjects also comprised a larger proportion in the BiCROS purchase group (52.4%) than in the withdrawal group (43.9%), although this was also insignificant (p=0.503). Males, even at old age, are often more inclined to social communication and relationship in South Korea. It is possible that this is why males are more likely to try and purchase BiCROS than females.
The proportion of the subjects who had a hearing loss duration of less than 15 years in the better-hearing ear was greater in the trial (50.0%) than in the non-trial (38.5%) group (p= 0.335) and in the purchase (52.4%) than in the withdrawal (43.9%) group (p=0.503); however, there was no significant difference. Patients with ASNHL and a shorter duration of hearing impairment might be more willing to try and purchase BiCROS.
There is still no consensus on the audiological indication for BiCROS use. When attending to a patient with ASNHL in the outpatient clinic, otologists have to consider multiple factors, including the audiological characteristics of the individual. Our data suggest the common range of hearing function for those who purchased BiCROS. The first and third quartiles of the PTA AC threshold in the better-hearing ear were 38.75 dB and 53.75 dB HL, respectively, with a median value of 45.00 dB HL. The first and third quartiles of the PTA AC threshold in the worse-hearing ear were 72.50 dB and 118.75 dB HL, respectively, with a median value of 100.00 dB HL. If the function of the better-hearing ear is too poor, a patient with ASNHL will not benefit from BiCROS use because the better-hearing ear cannot be aided by the hearing aid. If the function of the worse-hearing ear is relatively good, a patient with ASNHL may not have the need for hearing rehabilitation using BiCROS. In this regard, we suggest the range of hearing function of the buyers of BiCROS in this study to be a useful basis for the audiological counseling and selection of candidates for BiCROS use among patients with ASNHL.
The decision for the use of hearing aids in patients with ASNHL may possibly involve more audiological factors. For example, the decision for the use of BAHA systems was reportedly dependent on more than just the hearing benefits [21]. Even after an individual has elected to procure the hearing aid, the actual use of the device is still not guaranteed. In a previous Korean study, the daily usage time of BAHA or CROS hearing aids was not relevant to the patient satisfaction or the functional hearing level of the better-hearing ear [11]. The maintenance rate of BiCROS use may also be improved by proper fitting and close follow-up [20].
This study has several limitations. First, the number of the subjects included in our analysis was not large enough for passing the normality test. Further investigation involving more subjects is advised in the future. Second, patient satisfaction of BiCROS use was only inferred from the patients’ decision for purchase in our study; however, the actual daily usage time of the device or the actual functional hearing gain was not evaluated. Lastly, the decision for the trial or purchase of the device may also be influenced by the individual’s socioeconomic status, including the employment status/occupation, monthly income, or demand for social communication, all of which were lacking in our analysis.
Among the 78 subjects with ASNHL, 52 patients (66.7%) elected to try BiCROS, and 21 patients (26.9%) purchased BiCROS. In the selected candidates for BiCROS use, age, sex, and the audiological parameters did not influence the decision for the trial or purchase of BiCROS. Nevertheless, this result may be attributed to the fact that BiCROS was only suggested to selected patients with ASNHL based on the individual hearing function and need for hearing rehabilitation by an experienced otologist. The distribution range of the auditory thresholds for the subjects using BiCROS in this study can be a useful basis for the counseling of patients with ASNHL and selection of candidates for BiCROS use.
Notes
Conflicts of interest
The authors have no financial conflicts of interest.
Authors’ contribution
Conceptualization: Jeon Seong and Byung-Yoon Choi. Formal analysis: Jeon Seong and Byung-Yoon Choi. Methodology: Jeon Seong, Seung Koo Yang, Pilkeun Jang, and Byung-Yoon Choi. Supervision: Sang-Yeon Lee, Marge Carandang, and Byung-Yoon Choi. Visualization: Jeon Seong. Writing—original draft: Jeon Seong. Writing—review & editing: Jeon Seong, Seung Koo Yang, Sang-Yeon Lee, Marge Carandang, and Byung-Yoon Choi.