Can Auditory and Vestibular Findings Differentiate Vestibular Migraine and Meniere’s Disease?
Article information
Abstract
Background and Objectives
Besides evaluating the auditory and vestibular systems of patients with vestibular migraine (VM) and Meniere’s disease (MD), this study aimed to examine the clinical overlaps between these two conditions by detailed evaluation of the patient’s symptoms.
Subjects and Methods
The ears of the patients with VM and MD were evaluated and patients’ vestibular and auditory complaints were questioned particularly. Pure tone audiometry, vestibular evoked myogenic potential (VEMP) responses, and caloric test results were evaluated for objective measurements.
Results
The VM group had better air-conduction and bone-conduction threshold and speech reception threshold and speech discrimination score test values (p<0.05). Regarding the interaural N1-P1 asymmetry ratio, the cervical VEMP between the groups had significant differences (p=0.019). The MD group had more unilateral tinnitus and ear fullness complaints and canal paresis results (p<0.01). The VM group had more motion sickness complaints (p<0.01).
Conclusions
If only ears with hearing loss are evaluated; there was no significant difference between VM and MD, but regardless of hearing level or only the patients with normal hearing were evaluated, the VM group had better hearing levels. It should be considered that patients with VM may have VM-independent hearing loss, and patient complaints should be sufficiently detailed to make an accurate distinction from MD.
Introduction
Many studies have identified a significant overlap between the symptoms of vestibular migraine (VM) and Meniere’s disease (MD) [1]. These disorders have distinct pathophysiological mechanisms. In the case of VMs, one of the first proposed explanations for the condition is the vasospasm of the internal auditory artery, followed by the implication of the trigemino-vascular system [1,2]. Given the time spent by neurologists and ENT specialists with patients with headaches and vestibulopathy in clinical practice, further differential diagnostic studies are needed to better define and treat VM. In over 25% of patients, MD and VM co-morbidities. The prevalence of VM is approximately 1%, but it cannot be distinguished from MD if only the medical history and symptomology are known [3]. The biggest difference in the diagnosis of these diseases is the auditory symptoms in the diagnosis of MD. Although it has been reported in studies that there may be complaints of tinnitus accompanying vertigo attacks in VM patients, 25% of migraine patients also have hearing loss [4,5].
In this study, in which we examined the differences and similarities between MD and VM, unlike other studies, it was aimed to evaluate and compare the differences in hearing status as well as the vestibular evaluations and subjective complaints of the patients.
Subjects and Methods
Subjects
This retrospective study was approved by the ethical committee of the Istanbul Medipol University (No: E-10840098-772.02-2903). The requirement of informed consent was waived due to the retrospective nature of the study. The study included 42 VM patients (11 women) who applied to both Otolaryngology and Neurology Departments, and 56 MD patients (31 women) who applied to the ENT department. Those of patients were admitted to İstanbul Medipol Mega Hospital. VM patients whose neurological examination could not be completed and those with positive pathological findings on magnetic resonance imaging were excluded from the study. Subjective vestibular and auditory complaints of the patients were questioned in detail. Hearing thresholds, vestibular evoked myogenic potential (VEMP) responses, and caloric test results were evaluated.
The VM inclusion criteria are based on the “Diagnostic Criteria for Vestibular Migraine Proposed by Barany Society and the Third International Classification of Headache Disorders (ICHD-3) [6].” Patients with definitive diagnosis of vestibular migraine were included. The MD inclusion criteria are based on the “American Academy of Otolaryngology-Head and Neck Surgery Diagnostic Criteria for Meniere’s Disease [7].” Definitively diagnosed MD patients were included in the study. Data were collected during stable periods of patients’ symptoms.
Hearing evaluation
Air-conduction threshold (ACT) testing was conducted at 250, 500, 1,000, 2,000, 4,000, 6,000, and 8,000 Hz, and bone-conduction threshold (BCT) testing was conducted at 500, 1,000, 2,000, and 4,000 Hz frequencies. ACT and BCT were averages of 500, 1,000, 2,000, and 4,000 Hz. As a result of the average of the frequencies, 25 dB HL and below was accepted as normal hearing. Patients with values of 25 dB HL and above were considered with hearing loss [8].
VEMP testing
Cervical VEMP
Patients were instructed to turn their heads to generate the active neck rotation needed during the cervical VEMP (c-VEMP) test and for the recording of tonic background muscle activity. A recording electrode was located at the belly of the ipsilateral sternocleidomastoid muscle, a ground electrode was placed on the manubrium sterni, and a reference electrode was placed on the forehead. Reference latency values were determined as 13–23 ms at 100 dB HL. The p13 and n23 peaks occurring after 13 ms and 23 ms were defined as the first positive and negative peaks from the onset of stimulation, respectively. The p13 latency and peak-to-peak amplitude were defined as the difference between the p13 and n23 peaks.
Ocular VEMP
Patients were asked to fix their eyes at approximately 30° upwards in a superomedial gaze for the ocular VEMP (o-VEMP) recordings. This angle is reported to elicit the largest responses [9,10]. The recording electrode was placed over the contra-lateral inferior oblique muscle (centered under the pupil and 3 mm below the eye), the reference electrode was placed under the recording electrode, and a ground electrode was placed on the forehead. Responses averaged 150 stimuli. Reference latency values were determined as 10–15 ms at 100 dB HL. The first negative and positive peaks occurring between 10 ms and 20 ms after the stimulus onset were defined as n10 and p15, respectively.
Caloric testing
Both ears were stimulated separately with a 5-minute interval between tests. Jongkee’s formula was used to calculate unilateral weakness (UW) and gain asymmetry (GA) depending on the angular velocity values of the slow phase obtained by hot and cold stimuli of the ears. UW and GA values above 20% were considered abnormal.
Subjective complaints
Subjective tinnitus, ear fullness, and motion sickness complaints of the patients were stated verbally as yes and no by the patients. For tinnitus complaints, although the patients did not have a psychological disorder, it was questioned whether they could hear sounds at frequencies that did not exist outside. For ear fullness, patients were questioned whether they experienced a feeling of pressure in their ears as a result of the Eustachian tube being blocked or stopped working properly. For motion sickness, patients were asked whether they often experienced nausea and dizziness during the journey in the vehicle.
Statistical analysis
Statistical analysis was performed using the IBM SPSS Statistics software version 24.0 (IBM Corp, Armonk, NY, USA). Independent sample t-test was used for the statistical evaluation of hearing assessment and VEMP test results. Chi-square test was used for statistical evaluation of patient complaints and canal paresis results between groups. p<0.05 was determined as significance level.
Results
Demographic evaluation
A total of 42 VM patients and 56 MD patients were included in the study. When the ages were compared between the groups, no significant difference was found (p=0.064). When the sex was compared between the groups, a significant difference was observed, with more female patients in the MD group (p<0.001) (Table 1).
Hearing evaluation results
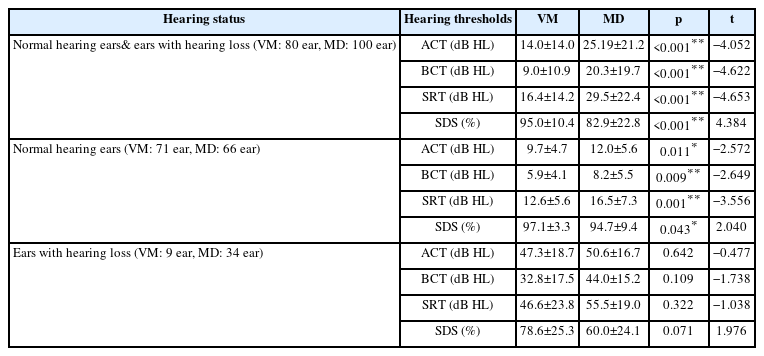
Patients’ hearing was evaluated by grouping them into different hearing states. When the ears were evaluated independently of the hearing threshold, a significant difference was observed between the groups. According to this result, VM group had better ACT, BCT, speech reception threshold (SRT), and speech discrimination score (SDS) values, on average of 11.19 dB HL, 11.3 dB HL, 13.1 dB HL, and 12.1%, respectively (p<0.05). In addition, when only ears with normal hearing thresholds were evaluated, the VM group had better ACT, BCT, SRT, and SDS test values (p<0.05) (Fig. 1). However, when the hearing thresholds of only ears with hearing loss were compared, no significant difference was observed between the groups (p>0.05) (Table 2).
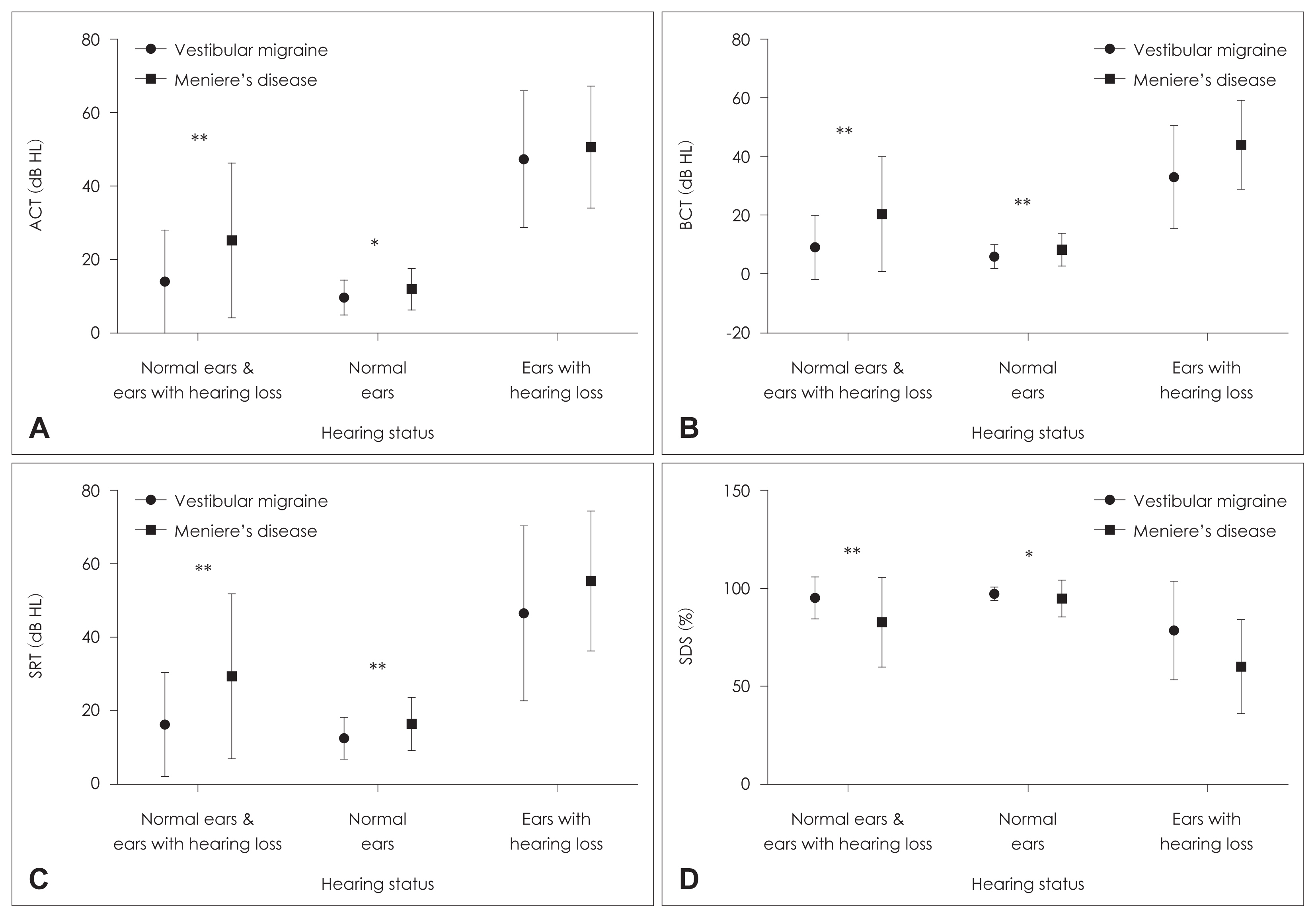
Comparison of hearing evaluation results according to different hearing status. A: Air-conduction thresholds (ACT). B: Bone-conduction thresholds (BCT). C: Speech reception thresholds (SRT). D: Speech discrimination scores (SDS). *p<0.05; **p<0.01.
VEMP evaluation results
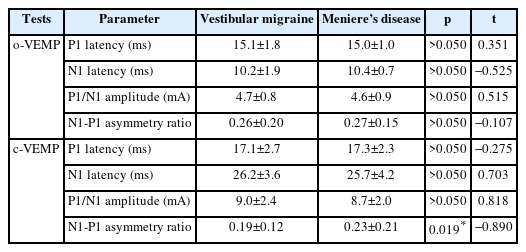
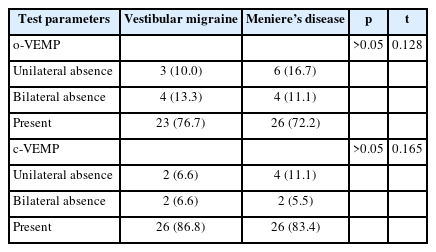
A total of 30 ears in the VM group and 36 ears in the MD group were evaluated with VEMP test. No significant difference was found between the groups regarding o/c-VEMP latencies of P1, N1, and P1/N1 amplitudes (Table 3). Regarding the interaural N1-P1 asymmetry ratio, a significant difference was found in c-VEMP between the groups (p=0.019) (Fig. 2). This ratio was 0.04 higher in MD group. When the patients were compared regarding the presence and absence of o/c-VEMPs, no significant difference was found between the groups (Table 4).
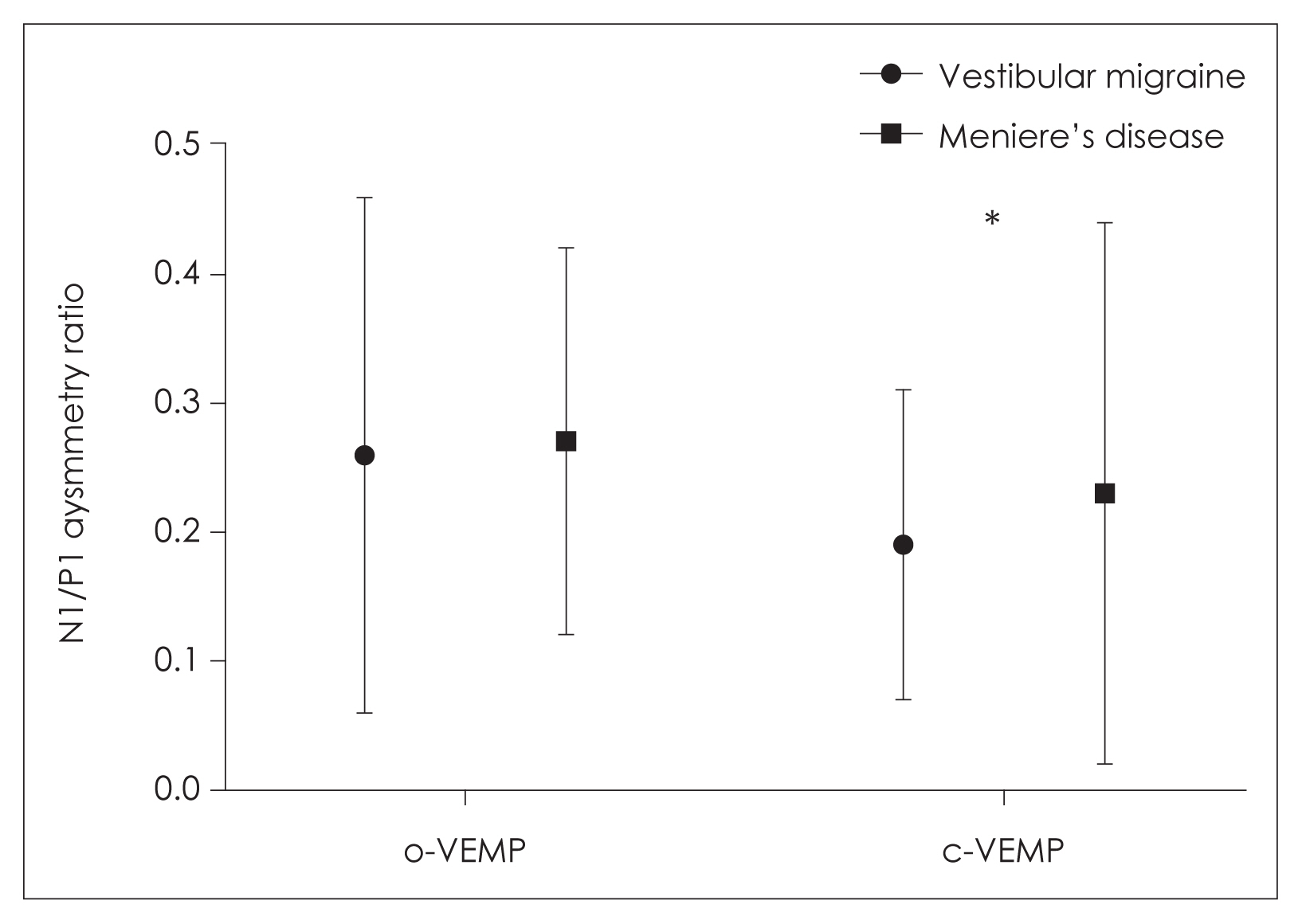
Asymmetry ratios of o/c-VEMPs. *p=0.019. c-VEMP, cervical vestibular evoked myogenic potential; o-VEMP, ocular vestibular evoked myogenic potential.
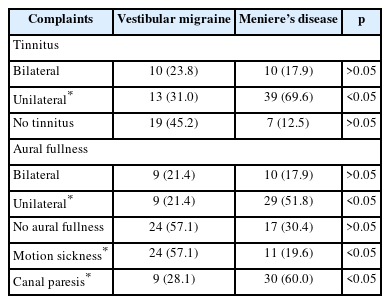
Tinnitus and ear fullness complaints
The tinnitus and ear fullness complaints of the groups were evaluated. It was noted that complaints of unilateral tinnitus and unilateral ear fullness were significantly higher in the MD group (p<0.05) (Table 5).
Motion sickness evaluation
A significant difference was observed in complaints of motion sickness (p<0.05). It was noted that complaints of motion sickness were significantly higher in the VM group (Table 5).
Caloric test results
There was a significant difference in canal paresis evaluated by caloric test (p<0.05). The finding of canal paresis evaluated with the caloric test was more common in the MD group (Table 5).
Discussion
VM may be one of the most difficult medical entities to diagnose because there is a certain overlap with MD in terms of its vestibular symptoms, although these diseases have different pathophysiological mechanisms. The idea that one may suffer from both diseases cannot be discounted, and therefore must be considered in clinical evaluation and treatment.
Hearing assessment studies have shown that MD patients have decreased hearing thresholds and speech discrimination scores, but these parameters may not sufficiently differentiate MD from coexisting with VM and MD [11]. In our study, when the hearing thresholds of the patients’ ears were evaluated, hearing test values of MD patients were worse than those of the VM group, regardless of hearing status among the patient groups. This may be attributed to the conclusion that, as expected, the presence of hearing loss was not found in the diagnostic criteria for VM as it was in MD. However, when the patients with hearing loss were compared with the groups, no significant difference was found between the groups. In this sense, it should be considered that VM patients may have a hearing loss independent of VM, and patient complaints should be sufficiently detailed in order to make a correct distinction from MD disease. The presence of accompanying ear complaints during attacks was a diagnostic criterion for MD patients, but not for VM patients. This may be an underlying reason why higher hearing thresholds were detected in the MD group. Due to hearing loss criteria in the definition of MD, lower speech discrimination scores and higher hearing thresholds may also distinguish MD from VM. With this result, it is important to pay attention to whether patients have hearing loss when evaluating them for VM and MD in a clinical setting.
In the previous studies, no significant was noted difference in latency and amplitude regarding the o/c-VEMP responses of VM and MD patients [12,13]. Another study identified significantly abnormal VEMPs in the MD group without indicating any o-VEMP or c-VEMP; however, the VEMP test results did not adequately distinguish between VM and MD [11]. In our study, higher asymmetry ratios of c-VEMP in MD group indicates the basic role of the saccule in the pathogenesis of MD. The histopathological analysis of MD can show that the saccule is more susceptible to endolymphatic hydrops than the utricle [14]. Different asymmetry ratios of c-VEMPs may indicate that the endolymph volumes in the saccule are regulated independently of each other in MD.
Although studies suggest that high levels of caloric asymmetry of MD patients may be a helpful diagnostic clue in differentiating between MD and VM [15], another caloric test results showed MD patients had low vestibular function, but no significant difference was observed in the caloric test between the two groups [16]. In our study, we found that the canal paresis of the MD group was significantly higher than that of the VM group in the caloric test.
Although the rate of bilateral tinnitus was not different between the VM and MD groups, it has been reported that the incidence of bilateral aural fullness is higher in VM (33%) than in MD (19%) [11]. In a large VM population and cross-sectional study by Çelebisoy et al. [17], 40.5% of the patients had tinnitus and 32.3% had ear fullness. In our study, no significant difference was found between the two groups in terms of bilateral tinnitus and ear fullness, however in terms of unilateral symptoms, significantly higher rates were found in the MD group. Hearing levels in MD patients decline to an average of 50–60 dB over a period of 5–10 years, while hearing loss in VM patients remains mild and declines much slower than in MD patients [18]. Therefore, we believe that unilateral tinnitus and ear fullness symptoms may be more common in MD than in VM, which is in line with the patients’ complaints.
There was a higher significant difference was detected in VM in terms of motion sensitivity compliance. Migraine is generally considered to be a disorder associated with higher sensitivity to different sensory stimuli. Because of the stimulating effects on the vestibular nuclei, migraine-associated brainstem regions may influence vestibular symptoms. Also, it may be responsible for increased motion sickness in the interictal period of VM and vestibular symptoms during a migraine attack. Studies reported significantly higher scores in VM patients by questioning their history of motion sickness [11,17]. In our study, we observed that the patients in the VM group had 37% more complaints of motion sickness compared to the MD group. The susceptibility to motion sickness in patients with MD and VM may be due to thalamus hypersensitivity.
In conclusion, with this study, which was carried out to reveal the common and distinctive aspects of VM and MD, significant results were encountered between the groups in neuro-otological evaluations. Although it has been reported in previous studies that hearing evaluation results differ between VM and MD patient groups, results that may help other studies were obtained in this study. When only ears with hearing loss are evaluated in the hearing evaluation; it was shown that there was no significant difference between VM and MD, however, regardless of hearing level or when only normal hearing patients were evaluated, the VM group had better hearing. In terms of this issue, it was underlined that the hearing level of the patients should be taken into account in the evaluation of VM and MD patients. Higher rate of asymmetry in c-VEMP, canal paresis in the caloric test, higher tinnitus and ear fullness complaints in the MD group, and complaints of motion sickness in the VM group were other supportive findings that should be considered. In the differential evaluation to be made the qualities of the audiological evaluations that can reveal the difference between these diseases should always be taken in account.
Acknowledgments
None
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: all authors. Data curation: all authors. Formal analysis: Handan Yaman. Investigation: Burcu Polat, Mustafa Bülent Şerbetçioğlu. Methodology: all authors. Project administration: Handan Yaman. Resources: all authors. Supervision: Burcu Polat, Mustafa Bülent Şerbetçioğlu. Validation: Burcu Polat, Mustafa Bülent Şerbetçioğlu. Visualization: Handan Yaman. Writing—original draft: all authors. Writing— review & editing: all authors. Approval of final manuscript: all authors.