Test-Retest Reliability of Tone Burst-Induced 500 Hz Air-Conduction Masseter Vestibular Evoked Myogenic Potential in Healthy Individuals
Article information
Abstract
Background and Objectives
Masseter vestibular evoked myogenic potential (mVEMP) is a newly developed tool which is used to assess the vestibulo-trigeminal neural and saccular functioning pathways. Recently, this test was added to a full test battery for evaluating the brainstem of people with neurological disorders and other vestibular diseases. For any test to qualify as a standard test, the test must have high reliability across all testing windows. Hence, the present study focused on validating the reliability of mVEMP in a large population.
Subjects and Methods
The study included 50 healthy participants with normal hearing. All the participants were tested using mVEMP and underwent retest within a month after the initial test. All parameters (latencies, peak-to-peak amplitude, asymmetric ratio) were observed for both sessions. To determine the statistically significant differences between and across the sessions, non-parametric tests such as Mann–Whitney U and Wilcoxon signed-rank tests were used.
Results
The test-retest reliability of all parameters were observed. The reliability was fair-to-good for P11 and N21 latencies. The other parameters showed less significance. There were no significant differences in sex and ear comparisons between and across the sessions.
Conclusions
Our study demonstrated that the mVEMP is a reliable test which can be used to assess peripheral vestibular system dysfunction and neurological conditions.
Introduction
Vestibular evoked myogenic potentials (VEMPs) are the responses from the otolith organs to loud sounds, vibration, or galvanic stimulation [1]. There are two primary types of clinically utilized VEMPs. Firstly, there is cervical VEMP (cVEMP), which involves recording responses from the sternocleidomastoid muscle and serves to evaluate the function of the saccular pathway. Secondly, there is ocular VEMP (oVEMP), which records responses from the inferior oblique muscle and assesses the functionality of the utricle pathway [2].
When exposed to a loud sound, the cVEMP produces twophase response waves. These responses occur exclusively when the relevant muscles are actively engaged [3]. Typically, these response waves are identified as P13 and N23, comprising an initial positive or inhibitory peak followed by a subsequent negative or excitatory peak [4]. In the context of cVEMP, the level of muscle tension is a contributing factor; when the appropriate muscle tension is generated, it results in an increase in the wave’s amplitude [4]. Research conducted on diverse groups of individuals with various vestibular conditions has shown that the VEMP responses are influenced by the saccule. Robertson and Ireland [5] also reported similar findings, indicating that the P13 and N23 peaks originate from the saccule. Hence, the practical use of cVEMP lies in its ability to assist in distinguishing and diagnosing various vestibular disorders, including but not limited to Meniere’s disease, vestibular schwannoma, vestibular hypersensitivity disorder, superior semicircular canal dehiscence, vestibular neuritis, and more [4].
The oVEMP response consists of a two-phase surface potential, with negative and positive peaks typically occurring around 10 ms and 15 ms, respectively. To measure it, positive electrodes are positioned just below the infra-orbital margin, and a negative electrode is placed 1 cm or 2 cm below the positive electrodes. This measurement assesses the vestibulo-ocular reflex integrity and reflects the motor function of the inferior oblique muscles, primarily on the opposite side of the body [6]. In clinical practice, oVEMPs are employed to evaluate the performance of the utricle, which is connected to the superior vestibular nerve. Unlike cVEMPs, oVEMPs are contralateral reflexes, meaning that the response is recorded from the eye opposite the ear that was stimulated [1].
In addition to the sternocleidomastoid and inferior oblique muscles, VEMP responses can also be detected in other muscles throughout the body, including the gastrocnemius [7], trapezius [8], triceps [9], and masseter muscle [10]. Stimulation of the vestibular system at the end-organ level can induce a rapid inhibitory electromyographic (EMG) response in the active masseter muscles. Initially termed the vestibulo-masseteric reflex (VMR) as a bilateral P11/N15 biphasic wave following unilateral or bilateral transmastoid electrical stimulation [11], it has more recently been referred to as masseteric VEMP (mVEMP) [12]. High-intensity acoustic stimulation also elicits VMR, but the N15 wave may appear inconsistently as a small deflection in a simple P11/N21 potential or may not be detectable at all [10]. Subsequent research revealed that the P11/N21 potential results from two overlapping components: a short-latency, high-threshold P11/N15 wave, not discernible in the rectified EMG, and a longer latency, low-threshold P16/N21 wave, clearly visible in the rectified EMG as a brief period of EMG suppression [10]. Patients with cochlear damage exhibited only the P11/N15 wave, while those with vestibular lesions displayed a preserved P16/N21 potential only [13], confirming the vestibular origin of the P11/N15 wave (VMR) and the cochlear origin of the P16/N21 wave, now termed the acoustic-masseteric reflex (AMR) [14]. The VMR shares physiological characteristics similar to the vestibulo-collic reflex, as it does not rely on vestibular receptors but instead directly stimulates the vestibular nerve. The VMR is a bilateral, biphasic, and symmetric potential with polarity opposite to that of the stimulus [10]. To record it, the procedure involves positioning the positive electrode on the lower part of the masseter muscle, placing the negative electrode on the zygomatic arch, and having a common electrode over the forehead. The recording process maintains muscle tension at a level between 30% and 50%, which can be monitored through visual feedback on the screen. Recently, the mVEMP, which records the VMR, has been employed for the evaluation of pathological conditions [15,16].
Test-retest reliability
Test-retest reliability, or reproducibility, is a technique employed to assess the reliability of a testing instrument. This is done by administering the same test to an individual or a group of individuals using the same procedures but on separate occasions, which could be hours or days apart [17]. It is crucial to have evidence demonstrating that the measurements obtained can consistently provide dependable scores. Reliability is a significant factor in all aspects of health measurements [18]. Numerous studies have provided strong evidence indicating that the test-retest reliability of both cVEMP and oVEMP is highly commendable [6,19-23]. Since mVEMP has been integrated into a comprehensive test battery, there have been relatively few studies that have assessed its test-retest reliability [15,16]. It is crucial to gather data on the test-retest reliability of mVEMP. Only a few studies have examined the test-retest reliability of mVEMP, and its validation for a broader population has not been established. Therefore, the current study is dedicated to validating the reliability of mVEMP within a sizable population.
Subjects and Methods
This study was approved by the Institutional Ethical Committee of SRM Medical College Hospital and Research Centre (SRMIEC-ST0123-401). In this research, a total of 100 ears was included, gathered from a cohort of 50 healthy individuals. The overall mean and standard deviation for the group were 22.50 and 3.85, respectively. Among them, there were 25 females with a mean and standard deviation of 22.20 and 3.84, and 25 males with a mean and standard deviation of 22.80 and 3.92. All participants gave their informed consent. The selection criteria for participants were as follows: ages between 18 and 40 years; normal hearing ability; absence of other medical conditions such as thyroid issues, diabetes, or high blood pressure; no use of medication; no history of ear surgery or other medical complications; and no established diagnosis of neurological disorders. The exclusion criteria included individuals with affected mastication muscles or temporomandibular joint issues and those who reported vestibular complaints. All participants went through a hearing screening assessment, which included passing a pure tone audiometry test (with results indicating hearing sensitivity below 20 dB), as well as tympanometry and reflexometry to evaluate the functioning of the outer and middle ear and their reflex pathways. To assess reliability, each participant underwent the mVEMP procedure on two separate occasions.
The mVEMP test involved the use of 500 Hz tone bursts generated through air conduction with alternating polarity. These tone bursts were delivered at a rate of 5.1 per second through ER-3A insert earphones. The stimulus was presented on the same side as the recording at a level of 125 dB SPL, and a bandpass filter with a range of 0.3 Hz to 2,000 Hz (using a Blackman gating function) was applied, along with 200 sweeps. Electrode placement involved positioning the inverting electrode on the zygomatic arch, the non-inverting electrode on the lower third of the masseter muscle, and the ground electrode on the forehead. Muscle contraction was maintained with the aid of a built-in visual display of the EMG needle deflection, which provided visual feedback on the computer screen. Participants were directed to maintain their masseter muscle tension within a specified range of 30% to 50%, determined by their maximum voluntary contraction values, during the recording process. The positive and negative peaks (referred to as P11 and N21) of the mVEMP were consistently identified with good morphology and reproducibility. Various parameters including peak amplitude, waveform latency, peak-to-peak amplitude, and asymmetric ratio were then measured and recorded.
Data analysis was conducted using Statistical Package for the Social Sciences (SPSS) version 26 (IBM Corp., Armonk, NY, USA). The collected data were organized and examined using this software. Test-retest reliability was evaluated using the intraclass correlation coefficient (ICC). The interpretation of ICC values followed the classification established by Versino, et al. [23], where values exceeding 0.75 were considered excellent reliability, values between 0.41 and 0.75 indicated fair-to-good reliability, and values below 0.40 indicated poor reliability. Additionally, Mann–Whitney U and Wilcoxon signed-rank tests were employed for gender and ear comparisons within and across the recording sessions.
Results
The aim of the study is to examine the consistency of test-retest results in healthy individuals when measuring mVEMPs using air-conducted 500 Hz tone bursts. Prior to this, all participants underwent evaluations for hearing and behavioral tests to exclude the presence of vestibular disorders and neurological conditions. Subsequently, various parameters including P11, N21, peak-to-peak amplitude (both rectified and unrectified), amplitude asymmetry ratio, and EMG averages were evaluated during the mVEMP procedure. The retest was conducted with the same group of participants within a one-month timeframe, during which all the previously mentioned parameters were also documented.
Mean values of mVEMP test parameters
All participants exhibited a 100% response rate in both the initial session (session 1) and the retest session (session 2). Table 1 presents the mean, median, and standard deviation values for various parameters, including the latency of P11 and N21, peak-to-peak amplitude (both rectified and unrectified), EMG average, and asymmetric ratio, for both testing sessions.
The percentage differences in mean and median values between the two sessions were calculated, and the p-values indicating statistical significance were determined. The results indicated that there was less significance (p<0.05) in the differences for P11, N21, and peak-to-peak amplitude (both rectified and unrectified), while there was no significant difference (p>0.05) for EMG average and the asymmetric ratio.
Test-retest reliability of mVEMP
All 50 healthy individuals participated in the retest session, which took place within a month following the initial session. The ICC (a measure of relative reliability) for P11 and N21 latency was determined to be in the fair-to-good range (ICC=0.40 to 0.75). Although there was consistency between the test and retest results, statistical differences were observed in the mean values of peak-to-peak amplitude (both rectified and unrectified), EMG average, and asymmetric ratio between the two testing sessions (p<0.05). However, it is important to note that these differences did not surpass the minimum detectable difference (MDD) threshold values, except in the case of peak-to-peak amplitude (rectified). In addition to assessing relative reliability, absolute reliability between the sessions was also examined. Smaller coefficient of variation of measurement error (CVME) values indicate higher reliability, as depicted in Table 2. The lower standard error of measurement (SEM) scores for all parameters suggest high reliability, except for unrectified amplitude and asymmetric ratio, which have larger SEM scores, indicating lower reliability.
Effect of ear and gender comparison on mVEMP parameters
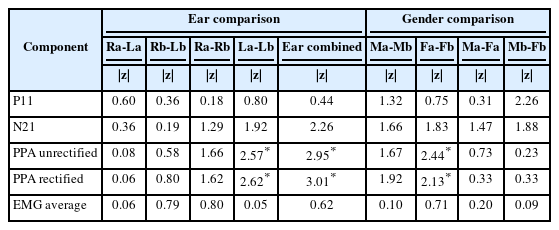
To discern significant differences in both inter-session and intra-session comparisons related to gender and ear, nonparametric statistical tests, specifically the Mann–Whitney U test and Wilcoxon signed-rank test, were conducted. The results of these comparisons, including the z-scores and p-values, are presented in Table 2. Regarding the ear-related findings, there was a significant difference observed when comparing the left ear across the sessions, as well as when comparing the combined data from both ears for peak-to-peak amplitude (both rectified and unrectified). In terms of gender-related findings, a significant difference was noted when comparing females across the sessions, particularly in relation to peak-to-peak amplitude (both rectified and unrectified).
Discussion
The primary objective of this study was to assess the test-retest reliability of mVEMP induced through air conduction. The study’s findings indicated a high level of consistency in mVEMP responses, with all 100 ears displaying consistent responses in both session 1 and session 2. The response rate was 100% for both sessions. These results align with a study conducted by Vignesh, et al. [15], which also reported a 100% response rate when comparing mVEMP results between two sessions. However, it is worth noting that a study by De Natale, et al. [14] reported slightly different response rates, with 84.7% for P11 and 67.8% for P16. These variations in reflex response rates could be attributed to differences in the electrode montage used in the respective studies [14].
Test-retest reliability of mVEMP
The current study’s overall test result demonstrates fair-to-good repeatability between two sessions, making it a viable indicator to employ in clinical populations. The positive (P11) and negative (N21) waves shows the fair-to-good test, retest reliability (0.41 to 0.75) between the sessions. The smaller CVME scores shows that the absolute reliability is higher for both sessions. The smaller SEM shows high absolute reliability between the sessions except unrectified and asymmetric ratio. Test-retest reliability was done by Deriu, et al. [13] using clicks evoked mVEMP shows the amplitude (raw and corrected) has high relative reliability (ICC) and more variable as the CV, SEM, and MDD scores are high, and for latencies and amplitude the ICC is good-to-excellent. A previous study of test-retest reliability using tone burst, results in excellent reliability for P11 latency, fair for N21 latency and shows no significant difference between both the sessions [15]. These results could be attributed to electrode positioning, and activation of the masseter muscles amongst participants could may play a major role. Since high intensities also stimulate both cochlea and vestibular, it is difficult to distinguish between the two-response reflex [13]. This can be a reason for the slightly increased P11 mean latency compared to the previous study.
Masseter VEMP is a low-cost, quick, straightforward, simple, and reliable test where it can be well tolerated. Due to this advantage of mVEMP, it can be used in any clinical setup which have axcess for recording the evoked potentials. As mVEMP share similar pathway as cVEMP and oVEMP, it can be used for diagnosing vestibular peripheral and central pathologies.
Effect of gender and ear comparison on mVEMP
The effect of the ear wise difference and gender comparison was observed for both the sessions. The comparison was done by the non-parametric test, Mann–Whitey U and Wilcoxon signed-rank tests for the related groups to identify the significance between the ears for both sessions. In the study for ear comparison, most of the parameter shows no significant difference for within and across session comparison, except left ear peak-to-peak amplitude (rectified and unrectified) for both the sessions. Eleftheriadou, et al. [24] did ear wise comparison using Wilcoxon signed-rank test, which results in some differences in p value between sessions, where the latencies and amplitude had no significant difference. The present study’s result is partially correlating with this study. The changes may be due to variations in the activation of the masseter muscle in left side compared to right, as well as individual to individual variations may also play a role.
In gender comparison the results showed that all the parameters had no significant difference between the sessions, except the female gender peak-to-peak amplitude (rectified and unrectified) for comparison of session 1 and 2. The peak-to-peak amplitude (rectified and unrectified) found to be higher for males compared to females. This difference can be due to more thickness of masseter muscle for males compared to females and large volume of trigeminal nerve for males compared to females [25,26].
Conclusion
The vestibular system is typically evaluated using two main tests: cVEMP and oVEMP. cVEMP helps assess the reflex associated with the sacculo-collic pathway, while oVEMP assesses the reflex related to the utriculo-ocular pathway. These tests are valuable for detecting various vestibular disorders, including conditions like Meniere’s disease, vestibular neuritis, vestibular schwannoma, Parkinson’s disease, and amyotrophic lateral sclerosis, among others. For individuals facing challenges in maintaining the required head position during cVEMP testing due to conditions like cervical spondylosis or other issues, a new test called mVEMP has been introduced. The mVEMP is a neurophysiological response that provides information about the vestibular system’s function, specifically the saccule and its pathways. When activated, the mVEMP is believed to involve the following physiological pathways: stimulation of the saccule. The saccule, one of the otolith organs located within the inner ear, is primarily responsible for detecting linear acceleration and gravity. In the context of mVEMP, it is stimulated, possibly by sound or vibration. The saccular stimulation triggers the vestibular nerve, which carries sensory information from the inner ear to the brainstem. The vestibular nerve is a component of the vestibulocochlear nerve (cranial nerve VIII). The vestibular nerve fibers carrying information from the saccule synapse in the vestibular nuclei within the brainstem. These nuclei process the vestibular signals, integrating information about head movement and spatial orientation. From the brainstem, the vestibular signals are transmitted to the motor neurons that control the masseter muscle. The masseter muscle, a jaw muscle, is involved in the mVEMP response, and its contraction is measured as part of the assessment. Surface EMG electrodes are typically placed on the masseter muscle to record the myogenic response triggered by the vestibular input. The recorded signal reflects the muscle’s contraction in response to the vestibular stimulation. Understanding these physiological pathways helps in interpreting the mVEMP response and provides insights into the integrity of the vestibular system, especially the saccule and its associated neural pathways. This information is valuable for clinicians who use mVEMP as a diagnostic tool to assess vestibular function. Keep in mind that the field of vestibular physiology is continuously evolving, and new research may contribute to a deeper understanding of these pathways. The recorded response latencies of masseter motor units imply the engagement of polysynaptic pathways in connecting the vestibular system to the trigeminal complex. The potential anatomical foundations for this VMR are explored [27]. For a test to be considered standardized, it should consistently produce reliable responses regardless of how many times it is administered. Numerous studies examining the test-retest reliability of both cVEMP and oVEMP have consistently demonstrated that these tests exhibit reliability across multiple testing sessions. Few studies have previously investigated the test-retest reliability of mVEMP, so this particular study sought to address this gap by conducting a comprehensive assessment of test-retest reliability across a significant and diverse population. The results of the present study indicate that the VEMP originating from the masseter muscle exhibits a level of reliability comparable to that of VEMP derived from cervical and ocular muscles. Therefore, mVEMP can be considered a viable diagnostic tool for evaluating vestibular functions, similar to cVEMP and oVEMP.
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Keerthi Ramesh, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja. Data curation: Keerthi Ramesh, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja. Formal analysis: Keerthi Ramesh, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja. Writing—original draft: Keerthi Ramesh, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja. Writing—review & editing: Keerthi Ramesh, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja. Approval of final manuscript: Keerthi Ramesh, Kumaran Thirunavukkarasu, Ramsankar Sujjuri Alagendraraja.
Funding Statement
None
Acknowledgements
The authors express their gratitude to the Department of Audiology and Speech Language Pathology at the SRM Medical College and Hospital and Research Centre, Kattankulathur, Tamil Nadu, India, for granting permission to conduct this study.