Relationship Between Chloroquine or Hydroxychloroquine Use and Hearing Disorders: A Systematic Review and Meta-Analysis
Article information
Abstract
Background and Objectives
Chloroquine and its analog hydroxychloroquine are derivatives of 4-aminoquinoline and are regularly used in the treatment of malaria and autoimmune diseases. Among the side effects of these drugs, alterations associated with the auditory system are frequently mentioned. Thus, the aim of this systematic review is to systematically review publications on hearing disorders and chloroquine or hydroxychloroquine use.
Materials and Methods
Inclusion criteria were observational or interventional studies on audiological assessment in participants who were using chloroquine or hydroxychloroquine. The methodological quality was independently assessed by two reviewers using the Meta-Analysis of Statistics: assessment and review Instrument. The certainty of the evidence was assessed using the GRADE tool.
Results
A total of 1,372 non-duplicate papers were screened, out of which 17 were included in the final qualitative synthesis, and 5 studies in the meta-analysis. The odds ratio for the two subgroups evaluated did not show significance with no heterogeneity between the effects observed between the different diseases (I2=0%) and obtaining the global estimate of 0.76 (95% confidence interval [CI]=0.41–1.39; p>0.05). Despite the inclusion of papers with different disease samples, the heterogeneity observed in the analysis was low (I2= 0%) and prediction interval (95% PI=0.32–1.80; p>0.05) remained close to that estimated by the CI (95% CI=0.41–1.39; p>0.05). The certainty of the evidence assessed by the GRADE tool was considered very low due to the risk of bias, indirect evidence, and imprecision.
Conclusions
The findings of this study suggest that the use of chloroquine/hydroxychloroquine is not associated with hearing disorders.
Introduction
Chloroquine and its analog hydroxychloroquine are derivatives of 4-aminoquinoline [1], which is an aromatic compound that has a flat central structure and basic side chain. However, its metabolism and excretion occur slowly, which can lead to accumulation in organs; when used by pregnant women, it can pass through the placenta [2].
These drugs are regularly used in the treatment and prevention of malaria and for the treatment of autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus (SLE) [3]. Currently, these drugs have gained prominence after authors that investigated their use as a possible treatment for COVID-19 verified the effectiveness and safety of these substances [4-6]. Regarding the use of these drugs, an international collaborative meta-analysis of randomized trials demonstrated that treatment with hydroxychloroquine was associated with increased mortality in COVID-19 patients, while no benefit was found with the use of chloroquine [7].
Chloroquine is considered more toxic when compared to hydroxychloroquine, and the use of chloroquine is decreasing as it triggers serious side effects, such as retinopathy and skin hyperpigmentation [8]. Among the side effects of these drugs, alterations associated with the auditory system are frequently mentioned. However, there is little scientific evidence regarding ototoxicity caused by the use of these substances [9].
To date, two systematic reviews have sought to investigate the association between the ototoxic effects of chloroquine or hydroxychloroquine [10,11]. In the publications, the studies included in the analysis referred to case studies, observing the need for a new systematic review that would expand the databases and information sources researched without imposing limitations regarding the period of publication of the studies, in addition, a search strategy that includes a greater number of references retrieved in your search. In addition, the tool used to assess the quality of the included studies in this review should be specified and its results should be quantitatively synthesized through meta-analysis.
Moreover, we observe that the actual damage caused to the auditory system by the use of these drugs is questionable, showing the need to carry out a systematic literature review to investigate this topic. Therefore, this systematic review aimed to answer the following focused question: “Is the use of chloroquine/hydroxychloroquine associated with hearing impairment?”
Materials and Methods
This systematic literature review was conducted following the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [12]. The protocol for this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42020180347).
Eligibility criteria
Eligibility criteria were determined based on the PECOS acronym:
Participants (P)
Studies performed in humans, with no restrictions on age, sex, and ethnicity were included. Experimental research (with animals or in vitro) and studies performed in patients with syndromes that present hearing alterations as a characteristic of the condition were excluded.
Exposure (E)
Studies in which the study population was exposed to chloroquine or hydroxychloroquine (at different periods and dosages) were included. Studies in which there was no such exposure were excluded.
Comparison (C)
The control group of the included studies should not have been exposed to chloroquine/hydroxychloroquine or treated with other medications (experimental-control).
Outcome (O)
The outcome of interest was to investigate the relationship between the use of chloroquine or hydroxychloroquine and hearing disorders. For this, the selected studies should have performed auditory tests (pure-tone audiometry, immittance measurements, auditory evoked potential, and otoacoustic emissions) in subjects who used these medications. Studies in which no audiological assessment was performed or in which diagnostic criteria were not validated were excluded from this review.
Study design (S)
Observational studies (cohort, case-control, and cross-sectional) and intervention studies (randomized, non-randomized, and pseudo-randomized clinical trials) which were found in their entirety were included, without limitation regarding publication date or language. Systematic or literature reviews, case reports, book chapters, letters to the editor, conference abstracts, and expert opinions were excluded.
Information sources and search strategy
Word combinations and truncations were adapted for each selected database. Embase, LILACS, PubMed/MEDLINE, Scopus, and Web of Science databases were used for searching. In addition, gray literature was searched through ASHAWire, Google Scholar, Open Grey, and ProQuest Dissertation and Thesis. An expert on the subject was also consulted, and reference lists of relevant papers were checked to identify complementary papers. The initial search was performed on April 25, 2020 and updated on April 23, 2021, by a researcher with experience in systematic review searches. Additional information on search strategies is provided in Supplementary Material 1 (in the online-only Data Supplement). The software EndNote (EndNote X7, Thomson Reuters, Philadelphia, PA, USA) was used to remove duplicate references.
Selection process
Study selection was performed in two phases by two independent reviewers (MRJ and JSO) to determine eligible studies. The first phase consisted of reading the titles and abstracts of the references retrieved by the search strategy. All papers that did not meet the eligibility criteria were excluded. The papers selected in the first phase were read in their entirety (phase 2), using the same eligibility criteria. Any disagreement between reviewers (MRJ and JSO) was discussed with a third reviewer (JBCB) in order to reach a consensus.
In both phases, the Rayyan website was used to shield the reading of the references and allow it to occur independently and confidentially (http://rayyan.qcri.org).
Data collection process
Two reviewers (MRJ and JSO) collected information from the studies selected for inclusion and the following parameters were extracted: study characteristics (author, year of publication, country, and methodology used in the study), sample characteristics (sample size, sex, age, and disease), assessments performed (pure-tone audiometry, immittance measurements, auditory evoked potential, and otoacoustic emissions), the characteristics of the results, and the main conclusions.
Three attempts were made by e-mail, one week apart from each other, to contact the authors and retrieve any unpublished data if the required data were incomplete.
Data items
The frequency of individuals who used chloroquine/hydroxychloroquine was extracted from the included studies, considering whether or not there was hearing loss.
Risk of bias assessment
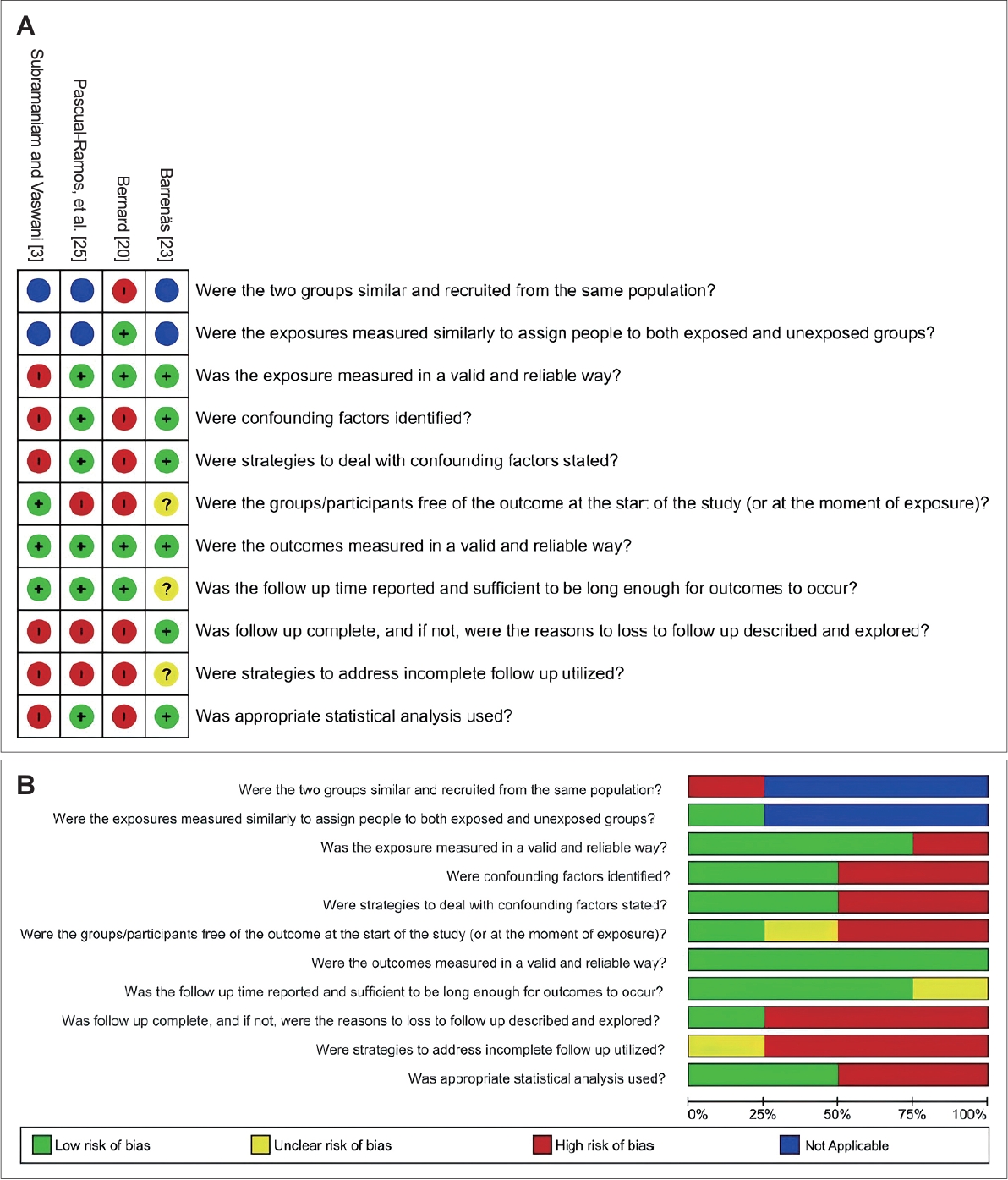
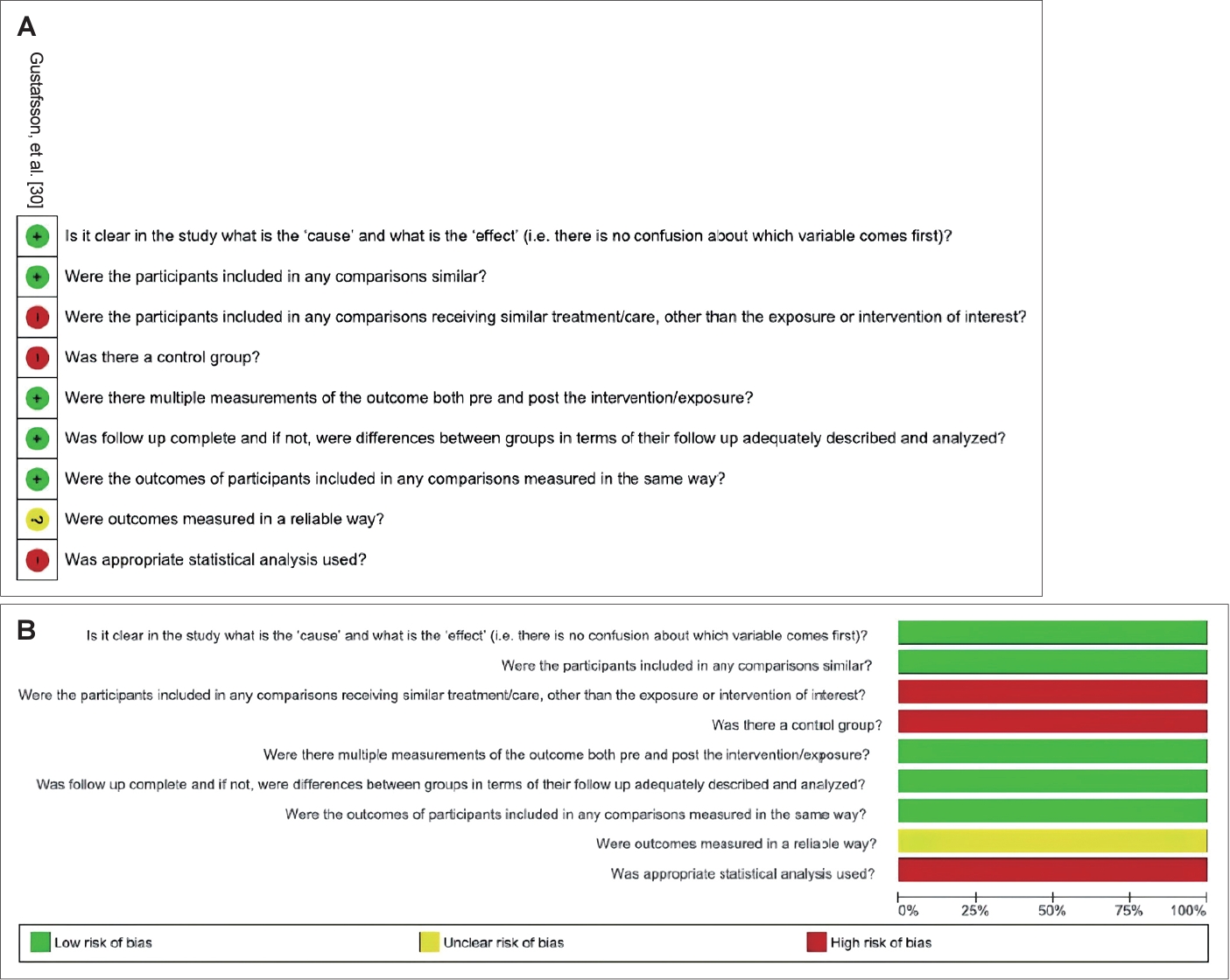
The methodological quality of the included papers was independently assessed by two authors (MRJ and JSO) using the Meta-Analysis of Statistics: Assessment and Review Instrument (MASTARI) [13]. This tool provided by the Joanna Briggs Institute (JBI) allows assessing the methodological quality of observational studies across nine domains. The MASTARI allows the following four response options: yes, no, unclear, or not applicable. The risk of bias, that is, variables that could “confuse” the results, was categorized for each of the studies as presenting a “high” risk of bias if the percentage of “yes” was lower than 49%; “moderate” if the percentage of “yes” was between 50% and 69%; and “low” if the percentage of “yes” was higher than 70% in the answers of the questions for assessing risk of bias. When there was any disagreement, it was resolved through a consensus meeting between the two first reviewers; if the disagreement remained even after the meeting, a third reviewer was involved for a final decision. The RevMan software, version 5.4 (The Cochrane Collaboration; https://revman.cochrane.org/info), was used to generate the figures of risk of bias.
Effect measures
To evaluate the results, the outcome of hearing loss was considered binary, thus making it possible to calculate the odds ratio between the use of chloroquine/hydroxychloroquine and hearing loss.
Synthesis methods
A method for random-effects meta-analysis using the statistical software RStudio version 1.2.1335 (RStudio Inc., Boston, MA, USA) was applied, with the studies being weighted by the Mantel-Haenszel method. Heterogeneity was calculated by the inconsistency index (I2) and the variance was obtained by calculating the tau2, which was estimated by the DerSimonian-Laird method. Sensitivity analysis was performed when a study judged to present a high risk of bias was included in the analysis, thus evaluating whether the inclusion of this study altered the estimates obtained. Confidence intervals of 95% were generated (95% CI) and the significance level was set at 5%. To analyze the influence of heterogeneity on the range estimates of the analyses, 95% prediction intervals (95% PI) were calculated for the estimated global effect. Analyses and the forest plot graph were generated using the R statistical software, version 1.2.1335 (RStudio Inc.).
Assessment of reporting bias
A minimum number of 10 studies included in the quantitative synthesis was stipulated to assess the risk of bias using the funnel plot and the Egger test. However, to avoid the likelihood of occurring publication bias, a broad search was performed in several databases, including a non-English-language database–LILACS, and gray literature.
Assessment of the certainty of the evidence
The certainty of the evidence was assessed using the Grading of Recommendations, Assessment, Development and Evaluation–GRADE [14] in all domains of risk of bias, inconsistency, indirectness, and imprecision. Quality was judged as very low, low, moderate, and high.
Results
Study selection
In phase 1, 1,914 references were found in all searches. After duplicates were removed, 1,372 papers remained for screening of titles and abstracts. After applying the inclusion and exclusion criteria, 31 papers were selected for full-text reading. One paper retrieved from the gray literature has been included for a full reading. After manually searching the reference lists and papers provided by the expert, 1 additional study was included. Therefore, 33 papers were retrieved for full reading (Fig. 1, flow diagram of literature search and selection criteria, adapted from PRISMA 2020). Sixteen of them were excluded for not meeting the selection criteria of this study (Supplementary Material 2 in the online-only Data Supplement).
Study characteristics
Seventeen articles were included, having as source the following countries: Brazil, Mexico, Argentina, Poland, Greece, Egypt, Sweden, Canada, India, Nigeria, Uruguay, and the United States. Among the studies performed with subjects exposed to hydroxychloroquine, the sample size ranged from 6 to 30 participants, aged between 19 and 69 years. In studies with chloroquine, the number of participants ranged from 5 to 79, with exposure to the drug ranging from 30 weeks of gestation (assessment performed on babies whose mothers had gestational malaria) to 85 years of age.
Seven studies analyzed the use of chloroquine and/or hydroxychloroquine in participants with SLE [15-21], 6 in participants with rheumatoid arthritis [20,22-26], 2 in participants with malaria [3,27], 2 without definition of the disease treated [28,29], and 1 in healthy participants [30].
As for the study design of the papers included, there were 16 observational studies (12 cross-sectional and 4 cohort studies) and 1 non-randomized clinical trial. All studies validated procedures for audiological assessment. In 15 studies, the assessment was performed with conventional pure-tone audiometry, 3 performed high-frequency audiometry, 1 study performed Békesy audiometry, 8 studies used acoustic immittance measures, and 7 studies used electrophysiological assessments, such as auditory brainstem response (ABR) in 5 studies and otoacoustic emissions in 4 studies. All the main characteristics of the studies included in the synthesis are available in Table 1.
Two studies were identified as potential studies for inclusion in this review, while, these studies cited only the p-value of the difference between the groups surveyed, they not identifying the number of participants who had hearing impairments in the groups exposed and not exposed to chloroquine [31] and hydroxychloroquine [32]. In a study, out of the total of 95 participants included in the sample, two individuals did not use hydroxychloroquine, who were not identified as being part of the group with or without hearing impairment [33]. Costa Branco Neves [34] reported that one of the participants affected by malaria and who used chloroquine had a comorbidity that was not identified, and it was not possible to verify whether the disease mentioned was related to hearing impairment.
Risk of bias in studies
The risk of bias was judged as low in 6 studies [17-19,21,22,24], moderate in 6 studies [16,23,25-27,30], and high in 5 studies [3,15,20,28,29] (Supplementary Material 3 in the online-only Data Supplement; Figs. 2-4).
Results of individual studies
There was disagreement in the literature regarding the association between the use of chloroquine/hydroxychloroquine and hearing loss. Most studies reported the lack of association between the use of hydroxychloroquine/chloroquine and hearing loss [15,19,22,24,25]. Roverano, et al. [15] reported that 16 patients using hydroxychloroquine (200 mg/day) had sensorineural hearing loss, but when comparing groups, there was no correlation between the presence of hearing loss and treatment with this drug. Gustafsson, et al. [30] observed that conventional and high-frequency audiometry did not reveal conclusive signs of ototoxicity in patients undergoing the use of chloroquine. In the study that evaluating 84 patients with systemic lupus erythematosus, there was a high prevalence of hearing loss, although not being associated with the use of antimalarials [21].
On the other hand, Kokong, et al. [28] and Obasikene, et al. [29] reported that among the drugs known to cause ototoxicity, chloroquine was the second or third drug most related to hearing loss, accounting for 14.1% of cases [29]. Mokbel, et al. [16] observed a significantly lower level of hearing in individuals who used azathioprine and hydroxychloroquine compared to individuals who did not receive this type of medication. In addition, it was observed that chloroquine increased the susceptibility to a temporary change in the post-exposure auditory threshold, which was more pronounced in young subjects with higher levels of dermal melanin [23].
Authors emphasized the recommendation of performing audiometric assessment before and after therapy with these drugs, especially in patients at risk of ototoxicity. However, ototoxic side effects are rare and reversible [3]. Bernard [20] reported that this reversibility occurs if the medication is suspended, but if the treatment is carried out continuously, the hearing loss may become irreversible.
Results of syntheses
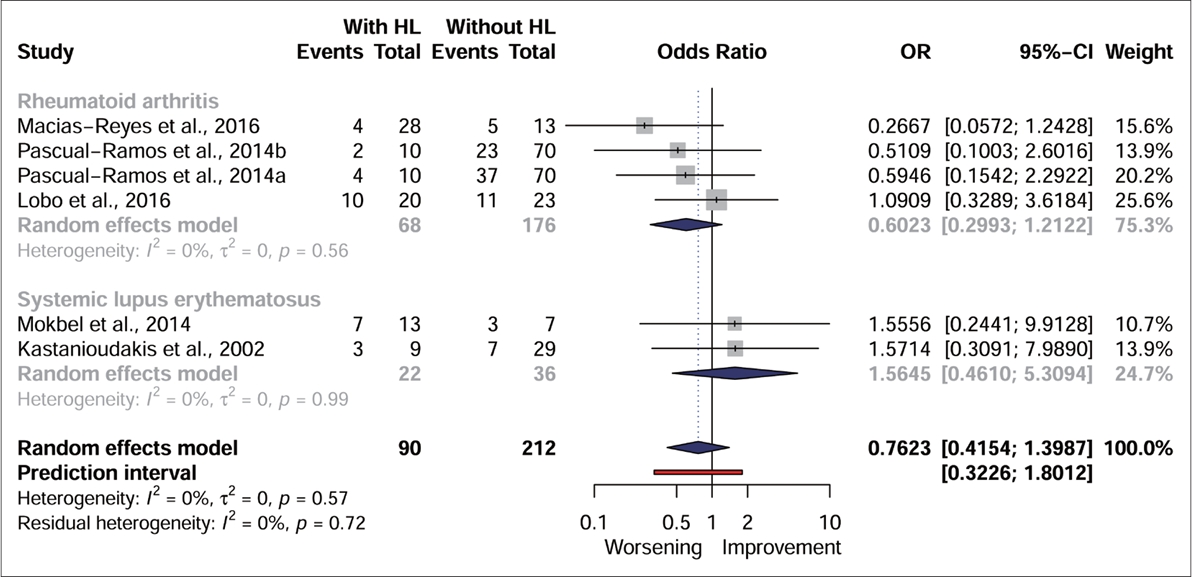
Five studies [16,19,22,24,25] were included for quantitative synthesis (Fig. 5). To assess the effect for each underlying disease when chloroquine/hydroxychloroquine was used, the analysis was divided into subgroups based on the diseases of the study sample. In the same sample, individuals diagnosed with hearing loss used the medication in the same proportion as individuals diagnosed without hearing loss, thus demonstrating the same odds ratio between using chloroquine/hydroxychloroquine and developing or not the assessed outcome (p>0.05). The odds ratio for the two subgroups evaluated did not show significance, with no heterogeneity between the effects observed between the different diseases (I2=0%) and an overall effect estimated at a chance of 0.76 [95% CI=0.41–1.39; p>0.05]. Despite the inclusion of papers with different disease samples, the heterogeneity observed in the analysis was low (I2=0%), and the prediction interval (95% PI=0.32–1.80; p>0.05) remained close to that estimated by the CI (95% CI=0.41–1.39; p>0.05).
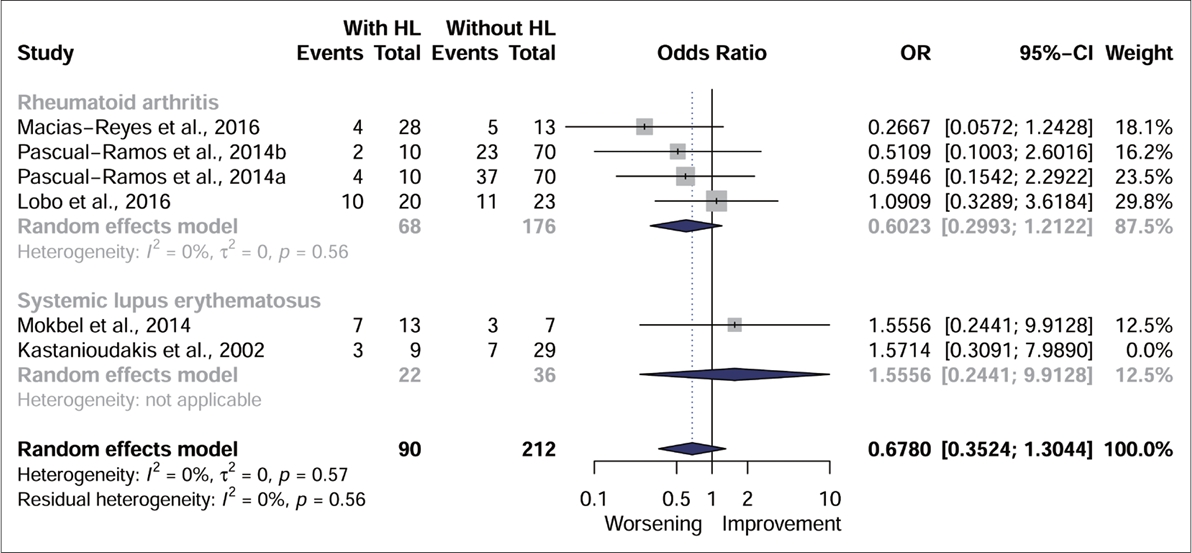
Even with the removal of the study judged to have a high risk of bias [19] from the analysis, the effect size remained similar, with no statistical significance (Fig. 6).
Reporting biases
As only 5 studies were included in the quantitative synthesis, it was not possible to assess publication bias using the funnel graph and the Egger test. However, the use of a broad search strategy and the inclusion of a non-English language database (LILACS), in addition to an extensive search in gray literature, decreased the probability of occurrence of this type of bias.
Certainty of the evidence
The certainty of the evidence assessed by the GRADE was considered very low (Supplementary Material 4 in the online-only Data Supplement). The main factors for decreasing the certainty of the evidence generated were the methodological limitations caused by the inclusion of two studies with moderate risk of bias [16,19] making it difficult to control confounding factors, indirectness (due to the design of the studies, it was not possible to analyze the groups that used or did not use chloroquine/hydroxychloroquine, and it was only possible to analyze participants with or without hearing loss who used the analyzed medications) and imprecision (wide range of prediction generated by the existing heterogeneity or by the low number of studies).
Discussion
Chloroquine and hydroxychloroquine are drugs that have been widely discussed recently due to their indication for the treatment of COVID-19 [4,6] and their adverse effects. Among the side effects related to their use, the risk of hearing impairment can be mentioned. In the studies included in this review, although the qualitative synthesis demonstrated disagreement regarding the association of the use of chloroquine/hydroxychloroquine with hearing impairment, the meta-analysis did not observe any association between these two variables, with the same chances of occurrence of hearing loss or not in users of these medications. These results suggest that, possibly, the audiological evaluation used by the literature included in this study is not efficient for detecting hearing disorders in people who use these medications.
Ototoxicity can be defined as the degeneration of cochlear and/or vestibular tissues, leading to the functional deterioration of these structures, due to the use of drugs or chemicals [35]. There are approximately 600 categories of medications associated with ototoxicity, in which symptoms can vary from hearing loss, tinnitus, ear fullness, dizziness, and vertigo to hyperacusis [36], with a high variability of symptoms due to genetic factors, comorbidities, and drug metabolization [37].
Confounding factors
There are studies in the literature that reported ototoxicity associated by chloroquine and hydroxychloroquine [26,28,29]. However, due to ethical issues, it is difficult to assess the pharmacodynamics of these substances in the auditory system of humans. Thus, these studies isolate other variables, such as the activity of autoimmune diseases [15,16,22,25], the combined use of these medications with other drugs [16,19,22,24,25], age [17,26], measurement of dose versus time of use of medications [17,19,22-25,28,29], and the predisposition of individuals to develop hearing disorders [38]. Therefore, the assessment of hearing loss caused by medication becomes complex due to the difficulty of excluding biases that may confound the results of the studies.
Five studies have related the use of chloroquine in samples with participants diagnosed with rheumatoid arthritis [20,22-25]. Three studies did not report medication dosage [22-24]. In one study, the dose of 150 mg/day was associated with methotrexate and/or sulfasalazine [25], while the dosage of 205 mg chloroquine was applied in combination with non-salicylate anti-inflammatory drugs in another study [20]. Three studies did not observe significant differences between the use of chloroquine in participants with or without hearing impairment [22-24], and one of these studies reported a high correlation between C-reactive protein and the use of methotrexate and sulfasalazine, without listing chloroquine as one of the factors associated with hearing loss [25].
Underlying disease
Except for one study that used chloroquine in healthy individuals [30], all the studies included in this review used the drugs to treat some diseases. Studies used chloroquine in subjects with malaria [3,27], rheumatoid arthritis [20,22-25], and SLE [17,18,20,21]. Due to the impossibility of discussing these data excluding the different diseases, the qualitative analysis of the results was carried out taking into account the use of medication for the treatment of different diseases.
It was observed that there was no association between the use of chloroquine/hydroxychloroquine and the presence or absence of hearing loss in the studies included in this meta-analysis, that is, the same odds ratio was obtained between using chloroquine/hydroxychloroquine and developing or not hearing changes. It is noteworthy the caution in performing the analysis by subgroups, according to the different diseases and the medication used. In studies in which the drug used was chloroquine, samples consisted of participants with rheumatoid arthritis [22,24,25], of which only one study cited a dose of 150 mg/day [25]. In research in which hydroxychloroquine was used, study participants were diagnosed with SLE [16,19] and one study reported a dose from 200 to 400 mg/day [16].
As for the use of chloroquine in subjects with rheumatoid arthritis, some studies suggested that sensorineural hearing loss may be associated with rheumatic disease activity [39,40], observing the prevalence of hearing impairment in 69.8% of participants in high-frequency audiometry and in 43.4% of participants in conventional pure-tone audiometry in the group of participants with rheumatoid arthritis [39]. Similar results were found, in which the frequency of hearing loss was significantly higher in the group of participants with rheumatoid arthritis when compared to the group without the disease [41].
Studies that related the use of chloroquine in participants with SLE [17,18,20,21], a study investigated hearing in children whose mothers used 250 mg/day of chloroquine during pregnancy, as well as in pregnant women with SLE who did not use the drug, verifying that none of the children who were exposed to chloroquine during intrauterine life had hearing loss [18]. There is no association between the use of chloroquine (this study did not show dosage) in combination with corticosteroids and hearing disorders in adults with SLE, only verifying a relationship between hearing loss and cardiovascular diseases [17].
A study [20] investigated the hearing of 13 subjects diagnosed with arthritis or SLE using chloroquine and found that although the pure-tone audiometry of all subjects in the sample was within the normal range, a change in ABR was observed for all study participants. After 12 to 16 months, only one subject had moderate bilateral sensorineural hearing loss, being the only one who needed to continue the use of chloroquine [20].
Some studies suggest that subjects with SLE are more likely to develop hearing alterations, suggesting that autoimmune diseases may be associated with hearing loss. A study noted that, in which 26.7% of the subjects with SLE presented hearing alterations in pure-tone audiometry, while 8.9% of the healthy subjects presented such alteration and related the used prednisolone, hydroxychloroquine, and methotrexate, but no relationship was confirmed between the types of medication used and hearing loss [42]. In another study with SLE, it was shown that 30.9% of subjects presented sensorineural hearing loss in conventional pure-tone audiometry and 70% had threshold changes at high frequencies [39]. A systematic review cited the action of SLE as a cause of structural changes in the inner ear [43].
Dosage and follow-up time
Although no association was observed between the use of chloroquine or hydroxychloroquine and hearing loss, one of the questions that should be critically analyzed is related to the measurement of the dosage and the duration limit of the treatment, so that hearing alterations do not occur, as well as to the methodology used in the studies that aim to investigate this association (12 of the total number of included studies carried out a cross-sectional analysis) and to the methods used to evaluate hearing alterations (most studies used pure-tone audiometry; 125 Hz to 8 kHz). Thus, the results of this meta-analysis do not intend to state that the use of chloroquine or hydroxychloroquine is not associated with hearing disorders. Instead, we aimed to draw attention to future researchers that may investigate the ototoxicity associated with these drugs to consider the importance of using a longitudinal analysis of the results by analyzing the maximum dose and time of use, so that these drugs do not interfere with the auditory system. Therefore, it will be possible to find the dose/time relationship that causes transient and/or permanent hearing disorders in subjects who use these medications.
In studies involving malaria, the chloroquine dose ranged from 25 mg dose/total, with 5 mg/kg/week (maximum dose of 300 mg) for 3 months [27] to an initial dose of 1,200 mg, followed by four doses of 600 mg every 12 hours [3]. A study applied to 34 pregnant women with malaria found a case in which the newborn had retrocochlear hearing loss and the mother contracted malaria in the first trimester of pregnancy. The baby, in addition to chloroquine, was also exposed to gentamicin for 9 days and required blood transfusion due to hyperbilirubinemia [27], which is characterized as a risk for hearing loss [44]. On the other hand, a study carried out with 30 adolescents/adults found altered thresholds in the frequencies of 8 kHz and 12 kHz, abnormal responses in evoked otoacoustic emissions, and alteration in the wave V of the ABR in two sample subjects of the sample after the use of chloroquine [3]. In a study carried out with chloroquine for the treatment of malaria, a higher proportion of severe and profound sensorineural hearing loss was observed among subjects treated with 250 mg chloroquine, including participants whose mothers were treated with the drug during pregnancy [34], which is not in agreement with the studies included in this review, where the proportion of subjects with hearing disorders was not significant [3,27].
In studies that investigated ototoxicity associated by various drugs, the dose of chloroquine was not mentioned, but a prevalence from 6.3% to 14.1% was observed, relating these drugs as the second and third main drug associated with hearing loss [28,29]. Studies that investigate ototoxicity generally use a database in which information related to confounding factors such as age, underlying diseases, comorbidities, time, and dose of medications are generally not considered.
A study carried out with a sample of 11 healthy individuals who used three doses of 300 mg of chloroquine observed that none of the subjects presented alterations in conventional puretone audiometry, although a change in hearing thresholds was observed in the high-frequency audiometry [30]. Ototoxicity initially affects the outer hair cells in the basal portion of the cochlea, causing changes in auditory thresholds at high frequencies [37]. Therefore most initial cases of hearing loss caused by ototoxic drugs are not identified by conventional pure-tone audiometry in the frequencies from 250 Hz to 8,000 Hz [45].
Among the studies that used hydroxychloroquine, 4 were used in samples of participants with SLE [15,16,19,21] and 1 in subjects with rheumatoid arthritis [26]. The use of hydroxychloroquine in participants with rheumatoid arthritis, the dose of the medication was not indicated and there was a significant difference between participants that had the disease and those using this medication, who presented a higher occurrence of hearing disorders [26]. It is noteworthy that in this study [26], the age of the participants ranged from 40 to 69 years, which can be a confounding variable in the study, since hearing loss may be related to hearing alterations and problems caused by aging. In a study with 23 users of hydroxychloroquine for the treatment of an autoimmune rheumatic disease [32], no significant differences were found in the hearing of users and non-users of the drug, suggesting that hydroxychloroquine was not responsible for the hearing changes.
In studies in which hydroxychloroquine was used in samples consisting of participants with SLE, it was observed in three studies that the daily dose of this drug ranged from 5.0 mg/kg/day [21], 200 mg/day combined with azathioprine, corticosteroids, methotrexate, and cyclophosphamide [15] to between 200 to 400 mg/day (mean 340±96.6) in combination with steroids and azathioprine [16].
In one study, hydroxychloroquine was used in combination with steroids, nifedipine, azathioprine, cyclosporine-A, methotrexate, and non-steroidal anti-inflammatory drugs, but the dosage of the drug was not mentioned [19]. In all studies in which hydroxychloroquine was used in samples diagnosed with SLE, there was no evidence of association between hearing impairment and the use of this drug [15,16,19,21], and only one study reported that the hearing thresholds in the frequency of 4,000 Hz in both ears of subjects who ingested azathioprine and hydroxychloroquine were significantly worse when compared to participants who did not use these medications [16].
Outcome measurement method
It is noteworthy that in some studies with chloroquine/hydroxychloroquine users, normal hearing thresholds were observed through pure-tone audiometry, although hearing alterations were observed in high-frequency audiometry or electrophysiological assessments (otoacoustic emissions or ABR). One of the possible hypotheses for this is the presence of hidden hearing loss, in which there are alterations in the synapses between the cochlear hair cells and the auditory nerve without changes in the auditory thresholds. These alterations can only be observed in wave I of the ABR or in electrocochleography [46,47].
Thus, the American Speech-Language-Hearing Association [48] and the American Academy of Audiology [49] recommend that, in addition to pure-tone audiometry at frequencies from 250 Hz to 8,000 Hz, audiometry should also be performed at high frequencies (9,000 Hz to 20,000 Hz), complemented by objective measures, such as otoacoustic emissions and tympanometry, and subjective measures, such as questionnaires. An ototoxicity monitoring programme proposed that tests that can measure the peripheral and/or central auditory function and the apical and basal turns of the cochlea should be performed, besides objective and subjective measurements [37].
Study limitations
As a limitation of this review, the difficulty of excluding variables that may confound the results presented in the studies can be mentioned, which occurred mainly due to the design of most of the included studies (observational). Thus, it is observed that the samples were mostly formed by convenience, making it impossible to select groups for factors such as age, use of chloroquine/hydroxychloroquine alone, users and nonusers of chloroquine/hydroxychloroquine, and other factors of exposure to aggressive agents. Finally, except for one study [30], this medication was used in samples with subjects with underlying diseases that may also be associated with alterations in the auditory system. In addition, chloroquine/hydroxychloroquine was also used in combination with medications identified as ototoxic, which are also factors that can confound the results found. Another limitation may be related to the very low level of evidence in the studies included in the meta-analysis, as these presented problems regarding the imprecision of the results found, indirect evidence, and the inclusion of papers with moderate risk of bias.
Conclusion
The results of this study suggest that chloroquine/hydroxychloroquine is not associated with hearing disorders, as the same odds ratio was observed between having or not having hearing loss in subjects exposed to these medications. It is suggested that due to the procedures used for auditory evaluation (pure-tone audiometry in the frequencies of 250–8,000 Hz), it was not possible to observe the association between chloroquine and hydroxychloroquine and hearing alterations, as most of the included studies presented less impact on these frequencies. However, we emphasize the need for auditory monitoring in subjects exposed to chloroquine and hydroxychloroquine with the use of tests that assess the functionality of the cochlear and auditory pathway functionality (electrophysiological procedures), as well as with high-frequency audiometry. These tests are more sensitive for the identification of hearing alterations associated with ototoxicity also in cases of normal hearing thresholds, enabling the early identification of transient or permanent hearing alterations in individuals using chloroquine and hydroxychloroquine.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.7874/jao.2023.00157.
Supplementary Material 1.
Database search strategy
Supplementary Material 2.
Excluded articles and reasons for exclusion (n= 16)
Supplementary Material 3.
Risk of bias for cross-sectional, cohort and quasi-Experimental studies, assessed by the Joanna Briggs Institute critical.
Supplementary Material 4.
Grading of Recommendations, Assessment, Development and Evaluation – GRADE
Acknowledgements
None
Notes
Conflicts of Interest
The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Maria Renata José. Data curation: Maria Renata José, Jéssica da Silva Ortega, Camila de Castro Corrêa, Karinna Veríssimo Meira Taveira, Cristiano Miranda de Araujo. Formal analysis: Maria Renata José, Camila de Castro Corrêa, Karinna Veríssimo Meira Taveira, Cristiano Miranda de Araujo. Investigation: Maria Renata José, Jéssica da Silva Ortega, Jordana Batista Correia Baran. Methodology: Maria Renata José, Jéssica da Silva Ortega, Camila de Castro Corrêa, Karinna Veríssimo Meira Taveira, Cristiano Miranda de Araujo. Project administration: Maria Renata José, Cristiano Miranda de Araujo. Supervision: Maria Renata José, Cristiano Miranda de Araujo. Validation: Maria Renata José, Débora Lüders, Claudia Giglio de Oliveira Gonçalves, Cristiano Miranda de Araujo. Visualization: Maria Renata José, Jéssica da Silva Ortega, Jordana Batista Correia Baran, Débora Lüders, Claudia Giglio de Oliveira Gonçalves, Bianca Simone Zeigelboim, Camila de Castro Corrêa, Karinna Veríssimo Meira Taveira, José Fernando Polanski, Rosane Sampaio Santos, Cristiano Miranda de Araujo. Writing—original draft: Maria Renata José, Jéssica da Silva Ortega, Débora Lüders, Camila de Castro Corrêa, Karinna Veríssimo Meira Taveira, Cristiano Miranda de Araujo. Writing—review & editing: Maria Renata José, Jéssica da Silva Ortega, Jordana Batista Correia-Baran, Débora Lüders, Claudia Giglio de Oliveira Gonçalves, Bianca Simone Zeigelboim, Karinna Veríssimo Meira Taveira, José Fernando Polanski, Rosane Sampaio Santos, Cristiano Miranda de Araujo. Approval of final manuscript: all authors.