 |
 |
- Search
| J Audiol Otol > Volume 25(3); 2021 > Article |
|
Abstract
Background and Objectives
Balance control is maintained in stationary and dynamic conditions, with coordinated muscle responses generated by somatosensory, vestibular, and visual inputs. This study aimed to investigate how the vestibular system is affected in the presence of an optical illusion to better understand the interconnected pathways of the visual and vestibular systems.
Subjects and Methods
The study involved 54 young adults (27 males and 27 females) aged 18-25 years. The recruited participants were subjected to the cervical vestibular evoked myogenic potentials (cVEMP) test and video head impulse test (vHIT). The cVEMP and vHIT tests were performed once each in the absence and presence of an optical illusion. In addition, after each test, whether the individuals felt balanced was determined using a questionnaire.
Results
cVEMP results in the presence of the optical illusion showed shortened latencies and increased amplitudes for the left side in comparison to the results in the absence of the optical illusion (p≤0.05). When vHIT results were compared, it was seen that the right lateral and bilateral anterior canal gains were increased, almost to 1.0 (p<0.05).
Conclusions
It is thought that when the visual-vestibular inputs are incompatible with each other, the sensory reweighting mechanism is activated, and this mechanism strengthens the more reliable (vestibular) inputs, while suppressing the less reliable (visual) inputs. As long as the incompatible condition persists, the sensory reweighting mechanism will continue to operate, thanks to the feedback loop from the efferent vestibular system.
Balance is the ability to stabilize our posture while on the move and stationery with the integration of needed inputs. Three basic mechanisms that provide input to this system are somatosensory, vestibular, and visual systems [1]. When a person sitting in a train, and watching outside from the window, both vestibular and somatosensory systems are signaling that the person is sitting still, whereas the visual system is signaling visually induced self-motion perception. This can be briefly described as sensory conflict. Although there is no definite description, conflict between what we perceive with the help of our sensory organs and reality are called “illusions” [2].
Cervical vestibular evoked myogenic potentials (cVEMP) enables the evaluation of the vestibular nerve function as well as otolithic organs and is described by Rosengren and Kingma [3] as the gold-standard. Video head impulse test (vHIT) is another widely used gold-standard objective vestibular test technique which is specifically designed for testing the function of each individual semicircular canal [4].
The aim of this study was twofold. One aim was to disturb the visual input using an optical illusion to evaluate the vestibular system and its role on maintaining balance. Second aim of the study was to understand the neurological pathways of the vestibular system and its interaction with the visual system. The future of research related to vection should rely on application of objective measures to complement traditional measures.
Twenty-seven females and 27 males participants between the ages of 18 and 25 years were included in the study. The participants did not report to have any neurological, psychological, or physical pathologies nor did they use any vestibulo- suppressant medication due to vertigo attacks in the last six months.
The participants were randomly divided into two groups, with the same number of males and females in each group. Since both tests used the neck muscles, to obtain more homogeneous results, we performed vHIT first on one group while performing the cVEMP test on the other group. We performed the first test in absence of and performed the second one in the presence of optical illusion.
cVEMP and vHIT were performed for each participant in the absence and presence of optical illusion. The cVEMP module in Interacoustics Eclipse (Interacoustics A/S, Middelfart, Denmark) was used for the procedure. Participants were tested sitting up with their heads turned towards their chins to contract their sternocleidomastoid muscles. 500 Hz tone-burst was presented at 100 dB SPL with insert earphones on the ipsilateral side. The participants’ neck muscle contractions were observed during each cVEMP test for optimum data and feedback was given to the person tested. To avoid low inter-test agreement two recordings with 200 sweeps each were taken from each side and their average value was used.
The vHIT was conducted with the VisualEyes Videonistagmography (Micromedical Technologies, Arlington Heights, IL, USA). For each semicircular canal 20 impulses were randomly presented to the participants by the same researcher to obtain the best data. Participants were advised to loosen up their necks and if they failed to do so a break was taken to maintain intended conditions.
The tests were performed with the inclusion of a projected optical illusion video on a smart white board in a blacked-out room to create an optimal environment (Supplementary Video 1 in the online-only Data Supplement). The optical illusions used were obtained from the provided video of Apthorp, et al’s study [5]. The participants were seated so their central as well as peripheral visual areas were covered with the given optical illusion for 120 seconds. Optical illusion stimuli were 1,000 randomly positioned blue dots per frame constantly turning around on a dark background. The blue dots were adjusted radially to move out from the center moving at a speed of 6 m/s in a virtual surface area of “30×30×80 m.”
While testing the cVEMP in the presence of optical illusion, the participants were seated so they can watch the illusions over their shoulder depending on which ear was being tested. For coherent testing during vHIT, the participants were seated 1 m from the target point for both testing conditions. The target point was put on the smart white board and its position was adjusted according to the height of each participant. The participants were also subjected to a subjective evaluation to see if they were feeling balanced during the tests. It was especially noted if they felt any kind of dizziness etc. in the presence of optical illusion.
Ethical committee approval was obtained from the clinical research ethics committee of Istanbul Medipol University (03.10.2018/534). Before the experiment, the study was explained to each participant and a written informed consent was obtained. All described procedures were conducted in accordance with the Helsinki Declaration of 2013.
Statistical Package for the Social Sciences version 22 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis. Normal distribution of the values was assessed using one-sample Kolmogorov-Smirnov test. Wilcoxon sign rank test was used to determine whether there was a significant difference between the data of two conditions. Significance value was taken as 0.05.
When P1 latencies were compared in the absence and presence of optical illusion, a statistically significant difference was obtained for the left side only (p=0.039) (Table 1, Fig. 1), while there was no statistically significant difference for the right side (p=0.253). There was also a statistically significant difference for left P1-N1 amplitudes (p=0.003) as well as left P1-N1 latencies (p=0.022), while there was none on the right side respectively (p=0.176, p=0.896) (Table 1, Figs. 2 and 3). There was no statistical significance in the asymmetry levels between the two conditions (p=0.291).
In the statistical analysis of the six semicircular canal vHIT gains, statistically significant difference was found for the right lateral canal (p=0.039) as well as for right anterior (p= 0.006) and left anterior canals (p=0.002). There was no statistically significant difference for the remaining semicircular canals: left lateral canal (p=0.458), right posterior canal (p= 0.070) and left posterior canal (p=0.931) (Table 2, Fig. 4).
The subjective evaluation for cVEMP showed that most of the participants (33 of 57 participants) didn’t feel any balance problems while watching the optical illusion, nine participants felt as if they were moving, four participants felt their surroundings were moving and 14 participants felt as if they were on a cloud. The subjective evaluation of vHIT showed that 38 of the 57 participants felt nothing while 11 of them felt as if they were moving themselves. Thirteen of the participants felt as if they were either on a cloud or dizzy.
Under normal circumstances, various sensory stimuli must be successfully integrated with each other in order for consistent movements to occur in the environment of the individual. However, if these sensory stimuli contradict each other, unexpected results can occur. “Vection” is the phenomenon where a false self-motion sensation is created with visual stimuli covering the visual field of a person. Some of the side effects of this sensation can be nausea, imbalance, blurred vision, and headache in individuals [6]. These side effects are defined as “cybersickness” or “visually induced dizziness” in the literature [6].
When a person is subjected to optical illusion, the stimuli gathered from visual and vestibular receptors contradict each other. As the contradiction of visual and vestibular stimuli increases, persons’ complaints and/or their feeling of self-motion become inevitable [7].
Stimuli from the senses are integrated with each other depending on their degree of their reliability within the central nervous system. Whichever stimuli the system deems more reliable has a more dominant (superiority in the hierarchy) place in the integration process [8]. If the reliability of a sensory stimulus decreases, the importance given to it in the integration process is also reduced, whereas another type of stimuli which is considered to be more reliable increased [9].
Integration zones of visual-vestibular stimuli in the cortex also contribute to this process. Vection is thought to activate, the middle temporal cortex (MT), cingulate sulcus visual region (CSv), precuneus, and parieto-insular vestibular cortex (PIVC) [10]. However, in another study, when individuals experience a sense of movement with optical illusion, a decrease in PIVC activity is observed [11].
When a person is watching an optical illusion, he recieves a false sense of movement originating from the visual system. However, simultaneously and constantly the inputs which are denying the movement comes from the vestibular system [12]. In this visual-vestibular conflict, the brain needs to habituate in order to eliminate the false sense of motion [7]. Weech and Troje [13] said that to resolve this sensory conflict, the effectiveness of vestibular stimuli that do not support the feeling that a person is moving is reduced. Thus, probably this is the way how conflict between visual and vestibular stimuli is eliminated.
In our study, we observed that the presence of an optic illusion activates the vestibular system, with increased amplitude and shortened wave latency in the cVEMP data. In addition, we observed increase in the bilateral anterior canals and right lateral canal vHIT gains (gains approached 1). This may also suggest that there is an increase in the reliability of the information obtained from the vestibular system. These results support the idea of a sensory (vestibular) reweighting mechanism actively working to maintain balance.
Sensory reweighting is a central nervous system mechanism that sorts through different inputs necessary (visual, somatosensory, and vestibular) to determine the ones more reliable in order to keep the balance [14]. If unreliable or unstable data are encountered, the impact of those inputs are reduced and the impact of more reliable inputs are reevaluated and likely enhanced [15]. For example, patients with vestibular dysfunction have incorrect vestibular inputs gathered from the peripheral organs.
Di Girolamo, et al. [16] reported that vestibulo-ocular reflex (VOR) gain decreases immediately after exposure to optical illusion. While in some studies with neuroimaging, in case of vection, deactivation of the vestibular cortex regions such as PIVC has been reported [11]. These findings support the suggestion that the vestibular cues are weakened when a sense of movement is triggered by a visual stimulus.
Harris, et al. [17] said that if the perception of distance was created with an optical illusion, in the presence of contradicting visual and vestibular cues, confidence in vestibular stimuli, not visual, is increased. In addition, it was observed that the importance given to postural control [18] or direction perception [19] increased when the reliability of visual stimuli decreased.
Responses in both semicircular canals and otolithic organs can stimulate neurons of the efferent vestibular system. In addition to the vestibular organs; inputs received from the visual system, pressure applied to the skin and passive movements of the extremities also provide information to the efferent vestibular system [20]. Although a direct link between visual stimuli and efferent vestibular system is unknown, given the complexity of the oculomotor pathways, these two systems are considered to be interacting.
Vestibular nuclei have been shown to be affected by stimuli from other systems other than vestibular stimuli, in the efferent vestibular system, mostly by stimuli from the visual system [21]. The most known neural pathway that transports visual stimuli to the vestibular nuclei is considered as an accessory optic system [22]. In addition, the vestibular nerve associated with the extraocular motor nucleus has been found to be stimulated by constant gaze, pursuit and saccadic eye movements [23]. Thanks to the constant feedback provided by the efferent vestibular system, received vestibular stimuli are constantly configured to provide the best data to maintain balance.
Changes in wave latencies and increased amplitudes in the cVEMP test performed in healthy individuals in the presence of optic illusion suggest that vestibular reflex pathways are also affected by the vestibular and visual cortex. Giving a visual stimulus causes physiological changes in the reflex arc which creates cVEMP response with a high amplitude [24]. Fowler, et al. [25] obtained higher cVEMP amplitudes in people with motion sickness and stated that there is a correlation between these two factors.
Gallagher, et al. [24] created an optical illusion (perception of movement) in adult participants by using VR goggles. They observed a significant increase in left cVEMP amplitude during exposure to optical illusion. They also observed that in the left cVEMP waves, the P1 latency was significantly shorter in the presence of optical illusion and the latency between the P1- N1 peaks was increased. As a result, they have stated that optical illusion affects the cVEMP reflex path, shortening latency and enlarging the wave amplitude [24]. This correlates well with our study, as we also found significant increase in left cVEMP amplitudes as well as earlier latencies in the presence of optical illusion compared to the values obtained in the absence of optical illusion.
In a study by Clarke and Schönfeld [26], an increase in unilateral vestibular reflex responses has been reported in various studies where optical and vestibular inputs differ from each other. Significant changes were observed in cVEMP asymmetry under conditions where the effect of gravity was changed. Decreased perception of gravity leads to changes in the functioning of the vestibular system. These changes may be similar to the absence of vestibular responses in the false sense of movement created with optical illusion. Consistent with this, they observed quite high cVEMP asymmetry in astronauts after spending some time in space however this asymmetry shifted to normal levels after 5-8 days of return.
Swathi and Sathish [27] evaluated the sacculocollic pathways using cVEMP test on individuals who were practicing dance for some time. Compared to the control group who have never had practiced dance at all, they observed a statistically significant decrease in P1 latencies as well as a statistically significant increase in the cVEMP amplitudes of the dancers. They concluded that the increase in amplitude and shortening in latency data may be due to plasticity in the sacculocolic pathways of the dancers.
The vestibular cortex network is thought to be asymmetrically located in individuals, with in right-handed individuals having a more developed vestibular cortex in the right hemisphere of their brain [28]. This difference in the right and left hemisphere vestibular cortex may be due to the interactions of vestibular and visual responses with each other. Schlindwein, et al. [29] showed that cVEMP testing is a reliable source for comparison of cortical activity between hemispheres. Kovács, et al. [10] observed greater activation in the right hemisphere MRI results than left in a study in which individuals assessed the movements of themselves and other objects. When all of these are evaluated together, differences in the vestibular cortex, vestibular processing pathway and vection can be taken into consideration among the reasons for higher left cVEMP amplitudes and earlier latencies in our study.
Clément and Reschke [30] measured VOR gains, phases, and speeds by exposing people to conditions that created motion sickness. They achieved a significant difference between VOR phase and velocity with motion sickness. Although they found no direct connection with VOR gains, the VOR mechanism is thought to be affected by the visual and vestibular system integration. Our study mimics the effects of motion sickness in the sense that it creates discrepancy. To our best knowledge, this is the first study in the literature to use vHIT data in order to compare the effects of presence and absence of optical illusion.
To prevent optokinetic nystagmus from forming we put a target for the participant to look at for VEMP too just like vHIT. The participants were instructed to look at this target and not watch, count, or follow the dots on the video. While conducting the vHIT, the clinician watched the participants eyes closely on the monitor and made sure that the eyes did not move from the target. Due to the participants instructions to not follow the dots and the constant supervision during the vHIT we presumed that optokinetic reflex did not take place. However, could not make sure of this in an objective way and this is a limitation to our study.
Subjective evaluations showed that in the presence of an optical illusion, people feel as if they are “moving” or “feeling on a cloud.” The fact that the data obtained from the visual and vestibular systems contradict each other support why people have felt this way. An analysis between subjects depending on their subjective evaluations were not conducted because of the low number of participants that reported feeling dizziness among other sensations. A further study with more focus on the reason of why participants did or did not feel dizzy etc. and its comparison is therefore suggested.
We still do not know how the afferent and efferent vestibular system works, especially the integration of different sensations in vestibular nuclei, cerebellum as well as the sensory reweighting mechanism. It would not be wrong to speculate that the effect of efferent system to the otolith organs and VOR to be different from each other. Meaning lateral and anterior canals’ gain increase might be due to the superior vestibular canal while there is no effect for this pathway to the otolith organs. However, when evaluating the results of the vHIT, while there was no significant difference in the posterior canals, there was increase in gains.
Lateral and anterior canals are stimulated by the superior vestibular nerve, while the posterior canal is stimulated by the inferior vestibular nerve. cVEMP, while stimulated primarily by the inferior vestibular nerve, is also stimulated partially by the superior vestibular nerve. The results made us think that aside from the sensory reweighting mechanism, the vestibular nerve may be playing a part in the discrepancies between the test and/or differences of gains regarding the semicircular canals. To get a better picture of the system and understand it better ocular VEMP test could have been conducted.
The results obtained in our study support the increase of reliability of the vestibular stimuli by suppressing the visual system. These findings suggest that the visual and vestibular systems are in constant interaction in order to maintain balance. Although the mechanisms of these two systems, especially at the cortical and efferent levels, are unknown; our study shows that these two systems work together in a constant state of interaction.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.7874/jao.2021.00080
Acknowledgments
We would like to thank participants for their patience and invaluable contribution to this study.
Notes
Author Contributions
Conceptualization: Şeyma Tuğba Öztürk, Mustafa Bülent Şerbetçioğlu, and Oğuz Yılmaz. Data curation: Şeyma Tuğba Öztürk and Kerem Ersin. Formal analysis: Şeyma Tuğba Öztürk and Kerem Ersin. Investigation: all authors. Methodology: all authors. Project administration: Şeyma Tuğba Öztürk. Resources: Şeyma Tuğba Öztürk and Kerem Ersin. Software: Şeyma Tuğba Öztürk and Kerem Ersin. Supervision: Mustafa Bülent Şerbetçioğlu and Oğuz Yılmaz. Validation: Mustafa Bülent Şerbetçioğlu and Oğuz Yılmaz. Visualization: Şeyma Tuğba Öztürk. Writing—original draft: Şeyma Tuğba Öztürk. Writing—review & editing: all authors. Approval of final manuscript: all authors.
Fig. 1.
Comparison of bilateral cervical vestibular evoked myogenic potentials P1 latencies in the presence and absence of optical illusion. *p≤0.05.
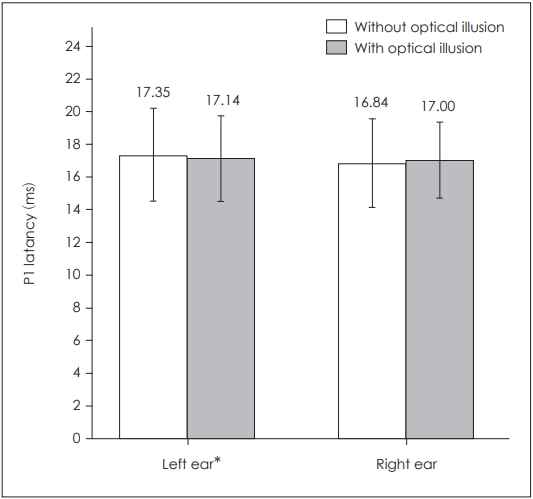
Fig. 2.
Comparison of bilateral cervical vestibular evoked myogenic potentials P1-N1 amplitudes in the presence and absence of optical illusion. *p≤0.05.
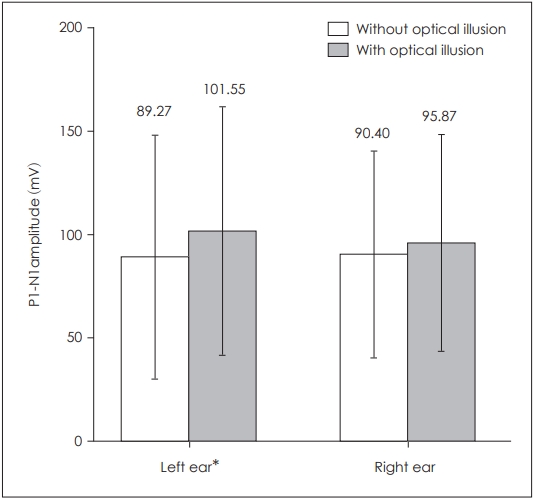
Fig. 3.
Comparison of bilateral cervical vestibular evoked myogenic potentials P1-N1 interpeak latencies in the presence and absence of optical illusion. *p≤0.05.
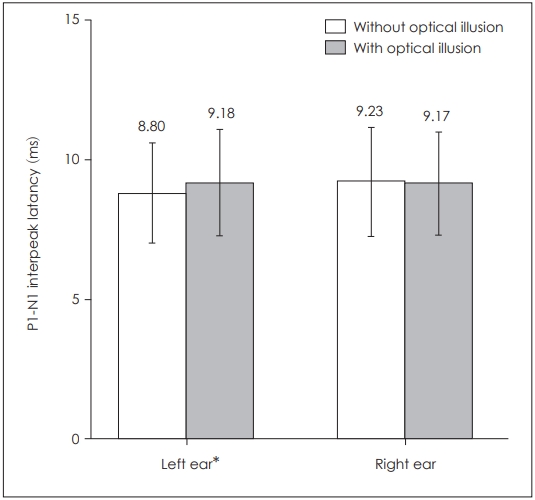
Fig. 4.
Comparison of vHIT VOR gains in the presence and absence of optical illusion. *p<0.05. vHIT: video head impulse test, VOR: vestibulo-ocular reflex.
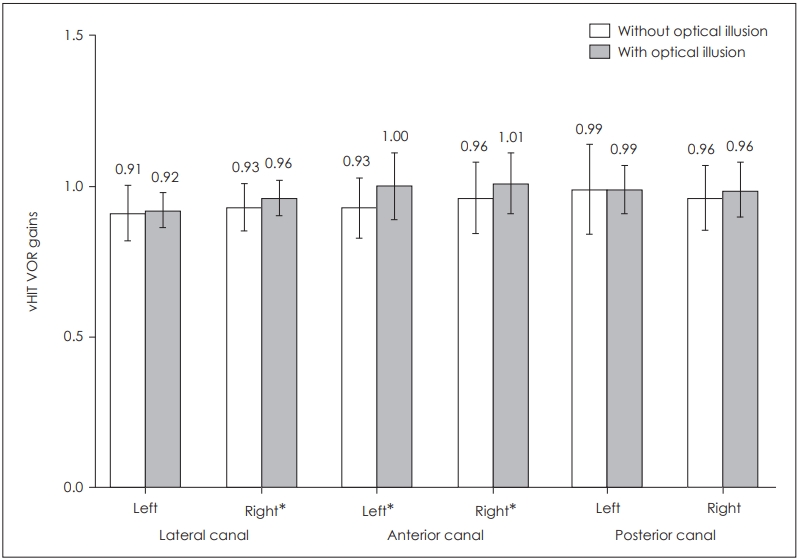
Table 1.
Comparison of cervical vestibular evoked myogenic potentials values in the absence and presence of optical illusion
|
Right ear |
Left ear |
|||||
|---|---|---|---|---|---|---|
| Absence of optical illusion | Presence of optical illusion | p-value | Absence of optical illusion | Presence of optical illusion | p-value | |
| P1 latency (ms) | 16.84±2.72 | 17.00±2.32 | 0.253 | 17.35±2.85 | 17.14±2.62 | 0.039* |
| P1-N1 latency (ms) | 9.23±1.96 | 9.17±1.82 | 0.896 | 8.80±1.81 | 9.18±1.91 | 0.022* |
| P1-N1 amplitude (µV) | 90.40±50.10 | 95.87±52.71 | 0.176 | 89.27±59.29 | 101.55±60.37 | 0.003* |
Table 2.
Comparison of video head impulse test VOR gains in the absence and presence of optical illusion
|
Right ear |
Left ear |
|||||
|---|---|---|---|---|---|---|
| Absence of optical illusion | Presence of optical illusion | p-value | Absence of optical illusion | Presence of optical illusion | p-value | |
| Lateral canal VOR gains | 0.93±0.08 | 0.96±0.06 | 0.039* | 0.91±0.09 | 0.92±0.06 | 0.458 |
| Anterior canal VOR gains | 0.96±0.12 | 1.01±0.10 | 0.006* | 0.93±0.10 | 1.00±0.11 | 0.002* |
| Posterior canal VOR gains | 0.96±0.11 | 0.99±0.09 | 0.070 | 0.99±0.15 | 0.99±0.08 | 0.931 |
REFERENCES
1. Dickman JD. Chapter 22 - The vestibular system. In: Fundamental Neuroscience for Basic and Clinical Applications (eds. Haines DE, Mihailoff GA). 5th ed. Philadelphia: Elsevier Inc;2018. p.320–33.
3. Rosengren SM, Kingma H. New perspectives on vestibular evoked myogenic potentials. Curr Opin Neurol 2013;26:74–80.


4. Alhabib SF, Saliba I. Video head impulse test: a review of the literature. Eur Arch Otorhinolaryngol 2017;274:1215–22.


5. Apthorp D, Nagle F, Palmisano S. Chaos in balance: non-linear measures of postural control predict individual variations in visual illusions of motion. PLoS One 2014;9:e113897.



6. Stanney KM, Kennedy RS, Drexler JM. Cybersickness is not simulator sickness. Proc Hum Factors Ergon Soc Annu Meet 1997;41:1138–42.

7. Akiduki H, Nishiike S, Watanabe H, Matsuoka K, Kubo T, Takeda N. Visual-vestibular conflict induced by virtual reality in humans. Neurosci Lett 2003;340:197–200.


8. Stein BE, London N, Wilkinson LK, Price DD. Enhancement of perceived visual intensity by auditory stimuli: a psychophysical analysis. J Cogn Neurosci 1996;8:497–506.

9. Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 2002;415:429–33.


10. Kovács G, Raabe M, Greenlee MW. Neural correlates of visually induced self-motion illusion in depth. Cereb Cortex 2008;18:1779–87.


11. Brandt T, Bartenstein P, Janek A, Dieterich M. Reciprocal inhibitory visual-vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain 1998;121:1749–58.


12. Stanney KM, Kennedy RS. Aftereffects from virtual environment exposure: how long do they last? Proc Hum Factors Ergon Soc Annu Meet 1998;42:1476–80.

13. Weech S, Troje NF. Vection latency is reduced by bone-conducted vibration and noisy galvanic vestibular stimulation. Multisens Res 2017;30:65–90.

16. Di Girolamo S, Picciotti P, Sergi B, Di Nardo W, Paludetti G, Ottaviani F. Vestibulo-ocular reflex modification after virtual environment exposure. Acta Otolaryngol 2001;121:211–5.


17. Harris LR, Jenkin M, Zikovitz DC. Visual and non-visual cues in the perception of linear self-motion. Exp Brain Res 2000;135:12–21.



18. Akizuki H, Uno A, Arai K, Morioka S, Ohyama S, Nishiike S, et al. Effects of immersion in virtual reality on postural control. Neurosci Lett 2005;379:23–6.


19. ter Horst AC, Koppen M, Selen LP, Medendorp WP. Reliability-based weighting of visual and vestibular cues in displacement estimation. PLoS One 2015;10:e0145015.



20. Holt JC, Lysakowski A, Goldberg JM. The efferent vestibular system. In: Auditory and Vestibular Efferents (eds. Ryugo D, Fay RR, Popper AN). New York: Springer;2011. p.135–86.
22. Barmack NH. Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull 2003;60:511–41.


23. Tomlinson RD, Robinson DA. Signals in vestibular nucleus mediating vertical eye movements in the monkey. J Neurophysiol 1984;51:1121–36.


24. Gallagher M, Dowsett R, Ferrè ER. Vection in virtual reality modulates vestibular-evoked myogenic potentials. Eur J Neurosci 2019;50:3557–65.


25. Fowler CG, Sweet A, Steffel E. Effects of motion sickness severity on the vestibular-evoked myogenic potentials. J Am Acad Audiol 2014;25:814–22.



26. Clarke AH, Schönfeld U. Modification of unilateral otolith responses following spaceflight. Exp Brain Res 2015;233:3613–24.


27. Swathi VM, Sathish KK. Influence of dance training on sacculocollic pathway: vestibular evoked myogenic potentials (VEMP) as an objective tool. J Evol Med Dent Sci 2013;2:7747–54.

28. Dieterich M, Bense S, Lutz S, Drzezga A, Stephan T, Bartenstein P, et al. Dominance for vestibular cortical function in the non-dominant hemisphere. Cereb Cortex 2003;13:994–1007.









