Introduction
Individuals with craniofacial, syndromic features are at high risk of having auditory neuropathy spectrum disorders (ANSD) [
1] as well as auditory processing disorders [
2] that affect perceptual abilities. Therefore, these individuals should undergo comprehensive audiological evaluation, including both behavioral and electrophysiologic measures, to assess the entire auditory pathway from the middle ear to the auditory cortex. This study examined a 53-year-old male, hereafter referred to as PR, with known mixed hearing loss, cognitive impairment, facial dysmorphology, and involvement across various systems. A comprehensive audiological behavioral and electrophysiological assessment of the middle and inner ear and the central auditory pathway was administered to identify the possible site(s) of lesion and to determine if ANSD was present. Psychological testing was conducted to evaluate PR’s current cognitive status, including the intelligence quotient (IQ). In addition, genetic screening was completed to determine a possible mutation for common syndromic hearing loss.
This case study of PR’s unique presentation adds to the knowledge base of craniofacial dysmorphologies associated with hearing loss. The study findings demonstrate detection of ANSD in the presence of mixed hearing loss, allowing for accurate diagnosis and appropriate intervention.
Case Report
History
Case history was drawn from previous medical records and a structured interview with PR and his caregiver. We placed special emphasis on audiologic and otologic history, related medical conditions, and family history.
Based on reports from PR’s mother, prenatal history was unremarkable; there were no complications during pregnancy and fetal development appeared to progress as expected. PR’s mother denies use of drugs or alcohol during the pregnancy. PR was born full-term via vaginal delivery. He was breech, with no other complications. His birth weight was 2.3 kg, which is considered low birth weight or small for a full-term gestational age [
3]. His mother reported that PR was late to meet various developmental milestones, such as sitting up, walking, and talking. PR’s mother reported that he sat up at 10 months, walked at 20 months, and began talking (1-2 words) at about 24 months. PR’s mother recalled that his pediatrician determined that he had delayed bone growth and he was diagnosed with LeggCalve-Perthes disease (avascular necrosis of the proximal femoral head) in his left hip. Karyotype at age four revealed normal male chromosomes. During childhood, PR appeared to have normal hearing sensitivity. Hearing loss was first diagnosed at approximately age 43. PR was diagnosed with moderate cognitive impairment, but the IQ was unknown. Given the craniofacial anomalies and cognitive impairment, PR had a computed tomography scan at age 18 for detailed anatomic descriptions and detection of associated intracranial lesions or anomalies of the central nervous system, and the results were normal. He had bilateral keratoconus and had a corneal transplant in the right eye in 1994, at the age of 31.
At the age of 43, PR has experienced bilateral chronic Eustachian tube dysfunction, resulting in recurrent serous otitis media in the left ear and bilateral high-frequency hearing loss. He has had several tympanostomy and pressure equalizing tubes in the left ear. He was first identified as having bilateral mixed hearing loss at 43 years of age and was fit with behind-the-ear hearing aids. Prior to the age of 43, history was negative for otitis media. When we evaluated PR in 2015, he was 51 years old, and his family reported a significant decline in his speech processing and understanding abilities. PR has a parental history of age-related and noise-induced hearing loss. PRs history is negative for noise exposure.
Procedures
A comprehensive evaluation was performed, including physical, audiological, psychological, and genetic evaluation.
Physical examination
PR is 160 cm tall, weighs 63.5 kg, and has a head circumference of 54.6 cm. His height is slightly below the 5th percentile while his weight is at the 5th percentile [
4]. His head circumference falls at the 25th percentile of head circumference for a height and gender matched group [
5]. PR displays hemispatial neglect toward his left side at the head area. He also displays musculoskeletal manifestations, including centralized obesity, weak muscle tone, abdominal hernias of unknown type, short stature (legs, arms, hands, head), uneven leg length most likely due to his Legg-Calve-Perthes disease, and flat feet. Clinical examination revealed facial dysmorphology, including: micrognathia (small jaw), microstomia (small mouth), smooth philtrum, large forehead, and low-set ears.
Audiological evaluation
Basic and behavioral audiological evaluation
Assessment was conducted twice in April 2015 and in May 2016. Otoscopic examination and 226 Hz tympanometry, using a Grason-Stadler Middle Ear Analyzer (GSI Inc., Eden Prairie, MN, USA), were conducted to assess outer and middle ear status. Behavioral audiometric test battery, including pure tone and speech audiometry, and hearing speech-in-noise and processing fast speech using QuickSIN (Etymotic Research, Elk Grove Village, IL, USA) test and a 40% Time Compressed Speech Test (TCST) at the most comfortable level, respectively.
Otoacoustic emissions (OAE), both distortion-product (DPOAE) and transient evoked (TEOAE), were collected, using a Natus Bio-Logic Navigator Pro Scout SPORT PC-based diagnostic OAE system (Natus Medical Inc, Middleton, WI, USA), to assess outer hair cell (OHC) function. OAE testing assessed frequencies from 500-4,000 Hz for click TEOAE and from 750-8,000 Hz for DPOAE. DPOAE responses were elicited with two “primary” frequencies (f
1 and f
2) at an f
2/f
1 ratio of 1.21, with f
2 frequencies varied from 750 to 8,000 Hz with the resolution of 3 points per octave. DPOAE level (at 2f
1-f
2) was measured with L1=65 dB SPL and L2=55 dB SPL. Responses were considered present if the signal-to-noise ratio (SNR) was ≥6 dB [
6].
Electrophysiologic audiological evaluation
Auditory brainstem response (ABR) to click and tone-burst (TB) stimuli, auditory middle latency response (AMLR), and cortical N1-P2 complex were recorded from both ears. All recordings were conducted using the Intelligent Hearing System-Smart-Evoked Potential (IHS-SmartEP; Intelligent Hearing Systems, Miami, FL, USA) that was calibrated following manufacturer specifications. All recordings were conducted using 2-channel recording, with disposable electrodes were placed on the PR’s head with the non-inverting electrode at the high forehead (Fz) for the ABR and the AMLR recordings and on the vertex (Cz) for the cortical N1-P2 complex recording. The reference, inverting electrodes were placed on each mastoid (M1 and M2), and the common ground electrode on the mid forehead (Fpz). Electrode impedances were equivalent between electrodes and were <5 kΩ. PR was quiet during ABR recording and, he was kept awake and alert during AMLR and N1-P2 recordings, respectively. The ABR was recorded to clicks and TB stimuli (1,000 and 2,000 Hz), condensation and rarefaction polarities. Stimuli were delivered at a rate of 21.1/s and an intensity of 90 dB nHL to an insert earphone (ER-3A). For TB ABR, 2,000 Hz frequency was chosen because it has the best hearing threshold with the least amount of ABG. Blackman window with a 2-1-2 envelope was used to enhance neural synchronization, specifically in individuals with neural desynchronization as in our case. Responses were amplified 100,000 times and filtered (10–3,000 Hz). Each ABR trace was derived from the average of 2,068 sweeps. The AMLR was recorded to an 80 dB nHL alternating click presented at a rate of 17.1/s. The window was set to 60 ms. The AMLR were amplified 75,000 times, filtered (3–1,500 Hz), and responses were collected to 1,530 sweeps. For the N1-P2 complex, 85 dB nHL, alternating clicks were presented at 1.1/s, responses were amplified (50,000 times) and filtered (1-30 Hz), and the window was set a 500 ms window. Each N1-P2 trace was collected to 100 sweeps. All responses, ABR, AMLR and N1-P2, were replicated, and the averaged responses were labeled.
Psychological evaluation
A comprehensive psychological evaluation was completed to assess intellectual ability, attention, language function, and IQ using the Stanford-Binet scale, 5th edition [
7]. This testing also assessed the general ability to reason, solve problems, visualize, and recall information in various forms. Testing was conducted by a licensed psychologist at the Learning Diagnostic Clinic on the university campus.
Genetic evaluation
Genetic evaluation was conducted by obtaining a family history and genetic testing with OtoSCOPE, a Next Generation Sequencing panel. The Molecular Otolaryngology and Renal Research Laboratories (MORL) at the University of Iowa conducted the OtoSCOPE genetic testing on a research basis. This genetic screening assesses 133 genes known to cause hearing loss, both non-syndromic and syndromic (for review, visit
https://morl.lab.uiowa.edu/genes-included-otoscope-v9). The MORL team completed the data analysis. The analytical sensitivity was >99% for regions sequenced with >10× depth of coverage; 99.63% of the 1.208 Mbp targeted were covered at >10× depth of coverage; 9,850,609 sequencing reads (100 bp, paired end) were aligned.
Basic and behavioral audiological findings
Otoscopy in the current study (2015 and 2016) revealed a clear view of the right canal and tympanic membrane (TM) and all landmarks were visible. The left ear showed a partially occluded view of the left TM, with a pressure equalizing tube placed.
Fig. 1 depicts annual pure tone thresholds and word recognition scores (WRS) from PR’s medical records from 2010-2014 and from the current study in 2015 and 2016. Pure tone thresholds were relatively stable from 2010 to 2012, with bilateral high frequency sensorineural hearing loss (SNHL) possibly due to early presbycusis. A significant decline in hearing occurred in the left ear, and a conductive component was noted in 2014, with progression of hearing loss mainly in the left from 2015 to 2016. Speech reception threshold (SRT) for the right ear declined slightly from 2010 to 2014 (15 dB to 25 dB), while left ear SRT declined significantly (30 dB to 55 dB). Results of WRS showed a similar trend from 2010 to 2016, with the right ear remaining stable (88-84%) but the left ear declining significantly from 76% in 2010 to 48% in 2016. A large SNR loss was evident on the QuickSIN test, bilaterally, with more SNR loss on the left (22.5 dB) than the right (12 dB). Also, PR has demonstrated a borderline TCST score on the right ear (78%) and a poor score in the left ear (64%) below the normal cutoff score of 82%.
When tested with standard 226 Hz tympanometry, results showed Jerger Type A tympanograms for the right ear and Jerger Type B tympanograms with normal ear canal volume for the left ear, consistent with otitis media with effusion (OME) build up behind an occluded pressure equalizing tube; similar findings were also obtained in 2012 and 2014. Acoustic reflex thresholds were absent ipsilaterally and contralaterally, bilaterally. The PI-PB function test in 2015 and 2016 showed a significant rollover of 0.4 for the right ear and 0.47 for the left ear, suggesting a retrocochlear site of lesion [
8]. Both TEOAEs and DPOAEs were absent at all frequencies, bilaterally. Absent OAEs typically indicate a cochlear loss due to OHC damage; however, PR’s extensive history of bilateral, recurrent middle ear issues and his current left mixed hearing loss should have impacted OAE results.
Electrophysiologic audiological findings
Fig. 2 displays the recorded click ABR. Click ABR produced no distinguishable waveforms with any polarity, and there was no evidence of cochlear microphonics (CM), bilaterally. In contrast, 2,000 Hz TB ABR to condensation and rarefaction polarities, as shown in
Fig. 3A, revealed presence of robust CM waves and poor ABR morphology for both ears. The presence of the CM waves was confirmed using two measurements. First, when we clenched the tube of the insert earphone (control condition) the CM disappeared (bottom traces of
Fig. 3A), confirming that the recorded responses were not artifact. Second, when the right 2,000 Hz TB ABR was recorded at different intensities (90, 80, and 70 dB nHL) the recorded CM waves at these intensities showed no latency shift, as shown in
Fig. 3B, indicating their intensity-independent, and this confirms that the recorded responses are truly CM waves and not a neural response [
9].
To evaluate the thalamocortical level of PR’s auditory pathway, click AMLR and N1-P2 responses were recorded in 2015 and 2016.
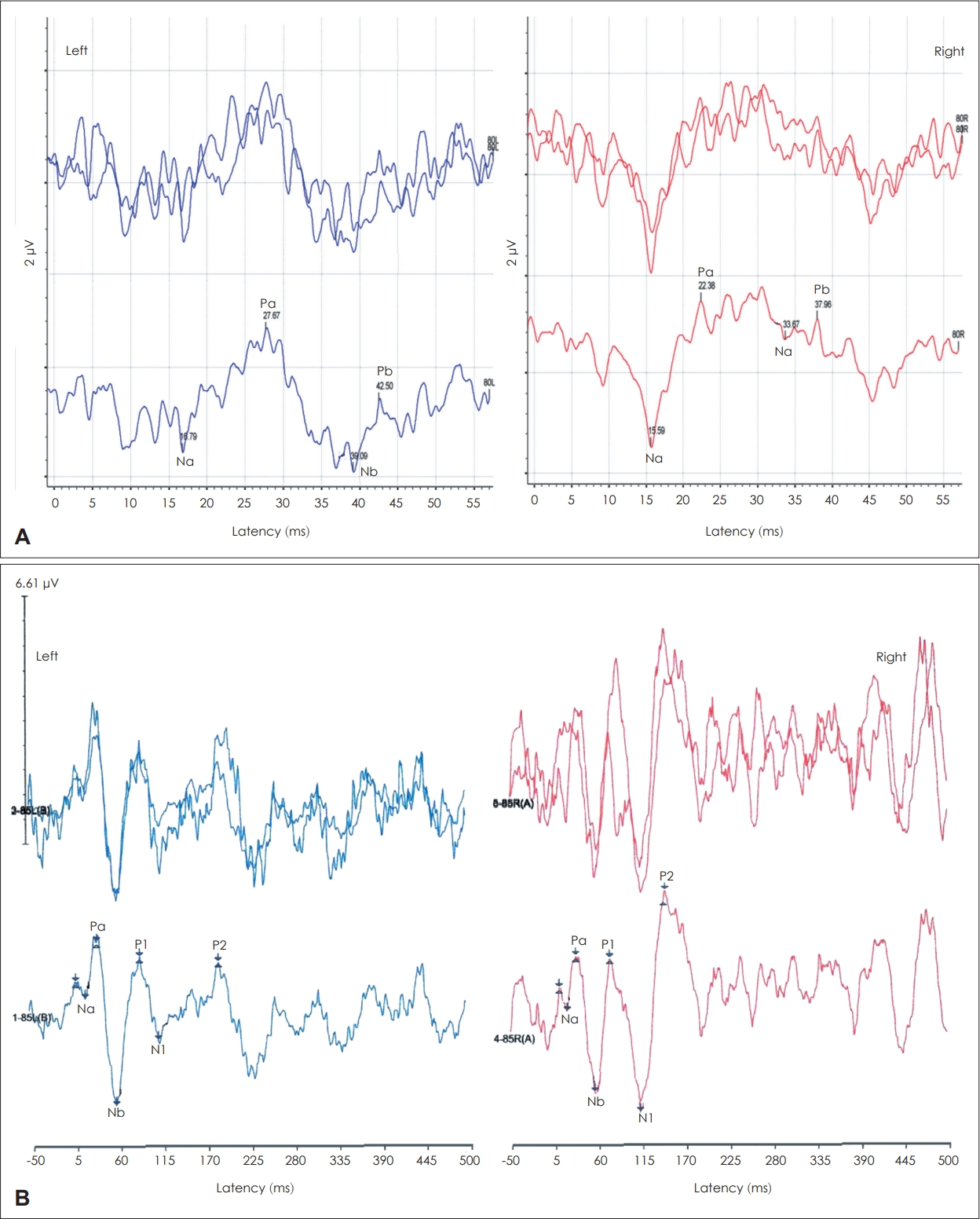
Fig. 4A shows the AMLR Na-Pa response in 2015 displaying grossly normal morphology and amplitude (2.9 μV) on the right. The left ear Na-Pa response was noisier, poorer, and slightly smaller (2.1 μV), with slightly delayed Pa latency (27.6 ms) compared to the right Pa latency (22.4 ms). Absolute latency of Na wave was within normal bilaterally (15.6 ms right; 16.8 ms left). The N1-P2 response was recorded, while an assistant was asking PR some questions to keep him awake and alert during the recording.
Fig. 4B shows that the response morphology, latency (N1=114 ms; P2=150 ms) and N1- P2 response amplitude were within normal on the right. In contrast, the left N1-P2 response morphology is abnormal with significantly smaller N1-P2 amplitude and delayed P2 latency (183 ms) than the right. These AMLR and N1-P2 findings are consistent with left auditory thalamocortical deficit.
Psychological evaluation
The psychological evaluation was conducted by a licensed psychologist. Results revealed that PR’s nonverbal IQ was 43, his verbal IQ was 46, and his full-scale IQ was 42. All scores fall into the moderately delayed range. The full-scale IQ of 42 is <0.1 percentile in comparison to age matched adults. These results indicate that nonverbal and verbal abilities are equally developed.
Genetic evaluation
Identification of specific gene mutations may further support the diagnosis of ANSD, and to help determine if the lesion is pre- or post-synaptic [
10]. A five-generation family pedigree did not reveal a mode of inheritance for PR’s syndromic features. The multidisciplinary Hearing Group at the University of Iowa, performed the OtoSCOPE next-generation sequencing panel and determined that no plausible variants, including either single nucleotide or copy number variants, were identifiable to explain the deafness phenotype in this subject.
Discussion
This study examined a subject suspected of having syndromic hearing loss to determine whether the hearing loss is due to ANSD and to identify the specific syndrome, if possible. Given the heterogeneous syndromic hearing loss and ANSD population [
11], a comprehensive audiological behavioral and electrophysiological test battery was used to assess the integrity of the central auditory pathway up to the level of the auditory cortex. In addition, genetic testing using OtoSCOPE screening for genetic mutations of hearing loss was performed. We also conducted psychological evaluation because our subject has cognitive impairment.
Audiological evaluation shows signs of ANSD
Results of speech audiometry revealed poor WRS that are disproportionate with the degree of hearing loss, and not related to cognitive impairment. Also, the significant rollover of speech recognition scores when speech was presented at loud sound is consistent with a possible neural hearing loss. The use of OAE testing with the absent responses was not helpful in our test battery to detect or rule out ANSD, because our subject had recurrent OME and repeated pressure equalizing tube placement. Therefore, recording ABR to both condensation and alternating polarity to assess CM response is recommended [
12]. Our finding of absent or indiscernible click ABR waves possibly due to presence of CHL and/or is indicative of a neural pathology. However, the lack of recording CM response in the absence of ABR responses to click stimulus cannot confirm the diagnosis of ANSD [
10]. On the contrary, recording CM with 2,000 Hz TB ABR supports the presence of ANSD bilaterally in our subject. To confirm the presence of the recorded CM response, absolute latency of the recorded responses at 2,000 Hz TB ABR showed no latency shift of the recorded waves with decreasing stimulus level, confirming present CM that is intensity-independent [
9]. In addition, when the insert earphone tube was clenched, as a control condition, the CM response disappeared. These findings indicate that PR’s SNHL component and other auditory manifestations are due to a syndromic ANSD and not a cochlear lesion. These findings highlight the importance of recording TB ABR when click ABR does not show CM responses in suspected cases with ANSD [
13].
The presence of abnormal left AMLR and N1-P2 response amplitudes and the delayed latency of the AMLR wave Pa and the P1 wave of the N1-P2 response supports the presence of abnormal left auditory thalamocortical and cortical pathway. These abnormal findings may be attributed to a combination of a post-synaptic ANSD due to lack of neural synchrony at the auditory nerve level and thus atypical onset precision neural input into the cortical level [
14], cognitive problem, and/or brain damage as a possible cause of PR’s hemifacial neglect. In addition, abnormal auditory cortical responses are biomarkers to predict poor behavioral speech perception in difficult-to-test individuals who cannot reliably participate in behavioral speech testing, and to determine the most appropriate amplification option in individuals with ANSD. Furthermore, the grossly abnormal left cortical recorded responses suggest that PR may have a problem discriminating rapid spectro-temporal transitions and auditory closure processing. These were demonstrated in our subject as manifested by significant speech rollover and severe deficits in hearing speech in less favorable listening environments such as fast speech as in TCST and background babbling noise as with QuickSIN test. On the other hand, PR’s poor scores on TCST supports processing issues involving both low-level peripheral processing, as shown with abnormal click and TB ABR findings, and high-level processing speed ability as shown with abnormal thalamocortical and cortical findings [
15].
Genetic testing did not detect mutations
Through genetic testing, identification of specific gene mutations may further support the diagnosis of ANSD. The OtoSCOPE DNA sequencing findings in this study showed no mutation in any of the 133 screened genes; however, SNHL is genetically extremely heterogeneous. According to the genetic team, although the subject’s SNHL is not due to a mutation in any of these 133 genes, the subject’s SNHL may be due to mutations in novel gene(s) or a mutation in a different gene that was not evaluated and/or age-related hearing loss. While the OtoSCOPE platform includes the most common genes known to cause non-syndromic and syndromic hearing loss, more than 110 genetic loci have been mapped and many hearing losscausing genes remain to be discovered. In conclusion, the use of TB ABR to condensation and rarefaction polarities and auditory cortical N1-Pe potentials should be clinically adopted as part of the test protocol when assessing individuals suspected of having ANSD, a lesion that has proven difficult to identify with genetic testing or with both OAE and click ABR, especially in individuals with recurrent middle ear issues. Because ANSD affects auditory processing and perceptual abilities, auditory cortical potentials should be recorded as a biomarker to also determine the best intervention course, and site-of-lesion. Though genetic assessments did not identify a particular syndrome or a mutation for ANSD, the comprehensive audiologic test results increased our confidence in labeling this a case of syndromic ANSD, associated with left thalamocortical and cortical deficits due to lack of neural synchrony and faulty neural input to the auditory cortex.
Acknowledgments
The authors would like to thank the research subject and his family for their participation in this study. The authors would also like to acknowledge Dr. Richard Smith and the Molecular Otolaryngology and Renal Research Laboratory team at the University of Iowa for providing genetic testing and interpretation of results. The authors would like to thank Ms. Rose Milcic, for helping with formatting and proofreading of the paper.
Fig. 1.
Puretone thresholds and word recognition scores (WRS) for PR show bilateral mild-to-moderate mixed hearing loss, sloping to moderate-to-severe high frequency loss. From 2010 to 2016, left ear thresholds progressed from mild-to-moderate mixed loss with fair WRS (76%) to moderate-to-severe mixed loss with poor WRS in 2014 and 2015 (52%–48%). WRS was not measured in 2010 and bone conduction thresholds were also not measured in 2010 and 2012.
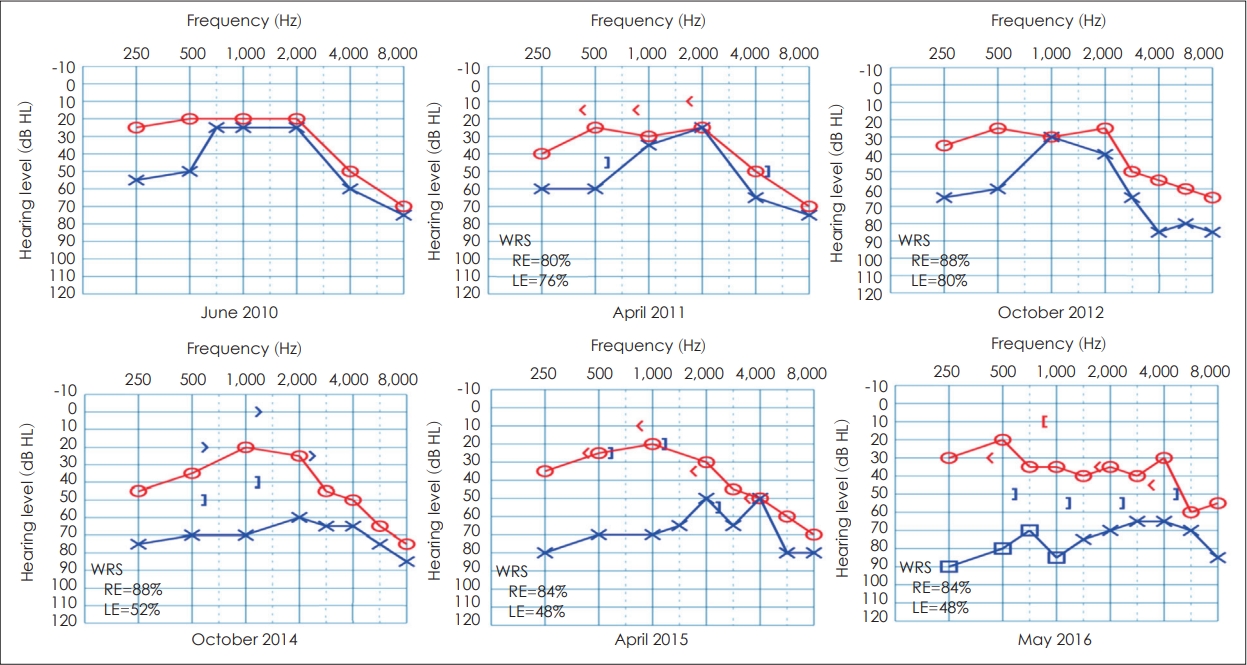
Fig. 2.
Click auditory brainstem response (ABR) recorded waveforms at 90 dB nHL and 21.1/s clicks for condensation, rarefaction, and alternating polarities from both ears. The click ABR morphology was poor, no distinguishable ABR waves with any polarity, and no evidence of cochlear microphonics, bilaterally. The abnormal click ABR findings on the left are due to the mixed hearing loss and the presence of otitis media with effusion (OME). For the ABR findings on the right, although the degree of hearing loss mainly at 4,000 Hz does not justify the absent responses to clicks, it is possible that previous history of recurrent OME may be the cause of the absent click ABRs.

Fig. 3.
Tone-burst (TB) auditory brainstem response (ABR) recorded waveforms at 2,000 Hz for condensation and rarefaction from both ears. A: 2,000 Hz TB ABR at 90 dB nHL and 21.1/s for condensation and rarefaction polarities from the left ear and the right ear. There were no distinguishable TB ABR waveforms, but there were present cochlear microphonics (CM) waves, bilaterally (two repeated traces and the averaged trace at each polarity). The control traces revealed disappearance of CM waves when we clenched the tube of the insert earphone, confirming that the recorded CM responses were not artifact. B: 2,000 Hz TB ABR recorded at different intensities (90, 80, and 70 dB nHL). Results showed no latency shift with decreasing intensity levels, confirming that the recorded waves are CM responses, not neural responses. These findings are consistent with auditory neuropathy spectrum disorders.
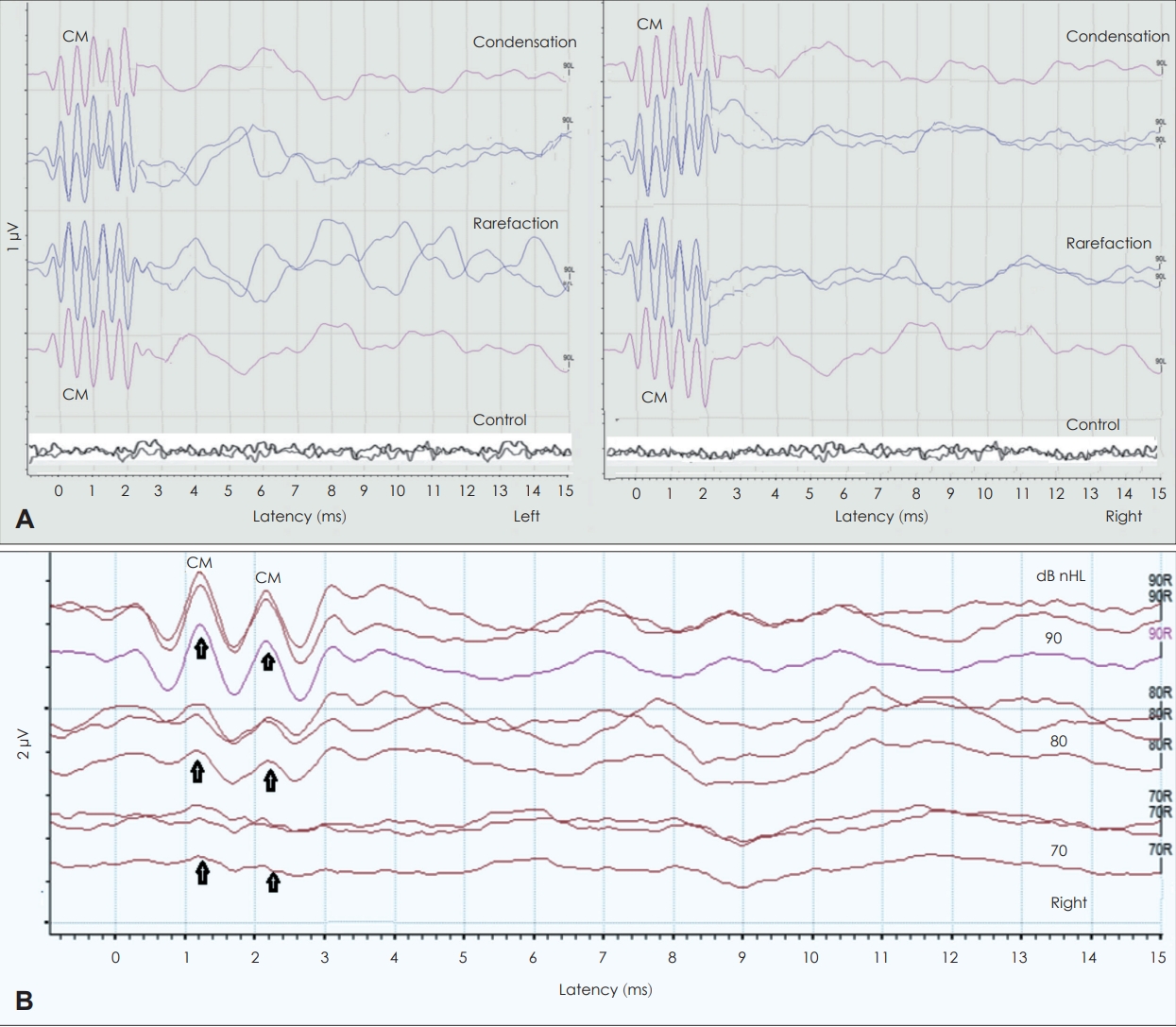
Fig. 4.
Suprathreshold click auditory middle latency response (AMLR) and auditory late-latency cortical (N1-P2) response recorded from both ears. A: The AMLR. The Na-Pa response displays grossly normal morphology and amplitude on the right, but noisier, poorer, with slightly delayed Pa latency on the left. B: The slow cortical N1-P2 response. The response morphology, N1-P2 amplitude, and N1 and P2 latencies are within normal on the right, but abnormal on the left (smaller amplitude and delayed P2 latency). Overall, these AMLR and N1- P2 findings are suggestive of an abnormality mainly involving the left thalamocortical pathways.
REFERENCES
1. Abdul Wahid SN, Md Daud MK, Sidek D, Abd Rahman N, Mansor S, Zakaria MN. The performance of distortion product otoacoustic emissions and automated auditory brainstem response in the same ear of the babies in neonatal unit. Int J Pediatr Otorhinolaryngol 2012;76:1366–9.


3. Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep 2017;66:1
4. Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007-2010. Vital Health Stat 2012;11:10–5.
7. Roid GH. Stanford-Binet intelligence scales. 5th ed. Itasca, IL: Riverside Publishing;2003.
8. Jerger J, Jerger S. Diagnostic significance of PB word functions. Arch Otolaryngol 1971;93:573–80.


11. De Siati RD, Rosenzweig F, Gersdorff G, Gregoire A, Rombaux P, Deggouj N. Auditory neuropathy spectrum disorders: from diagnosis to treatment: literature review and case reports. J Clin Med 2020;9:1074

12. Dallos P, Cheatham MA. Production of cochlear potentials by inner and outer hair cells. J Acoust Soc Am 1976;60:510–2.


14. Dimitrijevic A, Starr A, Bhatt S, Michalewski HJ, Zeng FG, Pratt H. Auditory cortical N100 in pre- and post-synaptic auditory neuropathy to frequency or intensity changes of continuous tones. Clin Neurophysiol 2011;122:594–604.


15. Pickora-Fuller MK. Processing speed and timing in aging adults: psychoacoustics, speech perception, and comprehension. Int J Audiol 2003;42 Suppl 1:S59–67.














