 |
 |
- Search
| J Audiol Otol > Volume 28(1); 2024 > Article |
|
Abstract
Background and Objectives
Subjects and Methods
Results
Notes
Author Contributions
Conceptualization: Hande Arslan. Data curation: Meltem Ãzdemir, Rasime Pelin Kavak, Kemal KeseroÄlu. Formal analysis: Hande Arslan. Investigation: Hande Arslan, Meltem Ãzdemir. Methodology: Hande Arslan. Project administration: Hande Arslan. Software: Kemal KeseroÄlu. Supervision: Murad Mutlu, Mehmet Hakan Korkmaz. Validation: Kemal KeseroÄlu, Rasime Pelin Kavak. Visualization: Murad Mutlu, Mehmet Hakan Korkmaz. Writingâoriginal draft: Hande Arslan. Writingâreview & editing: Hande Arslan, Kemal KeseroÄlu, Mehmet Hakan Korkmaz. Approval of final manuscript: all authors.
Fig. 1.
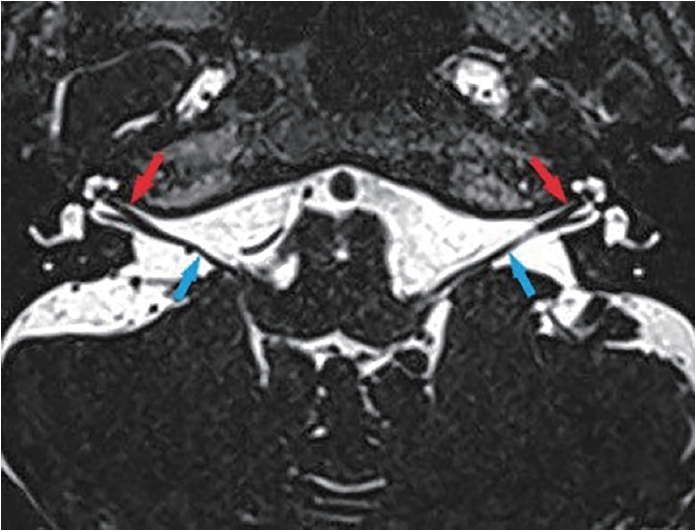
Fig. 2.

Fig. 3.
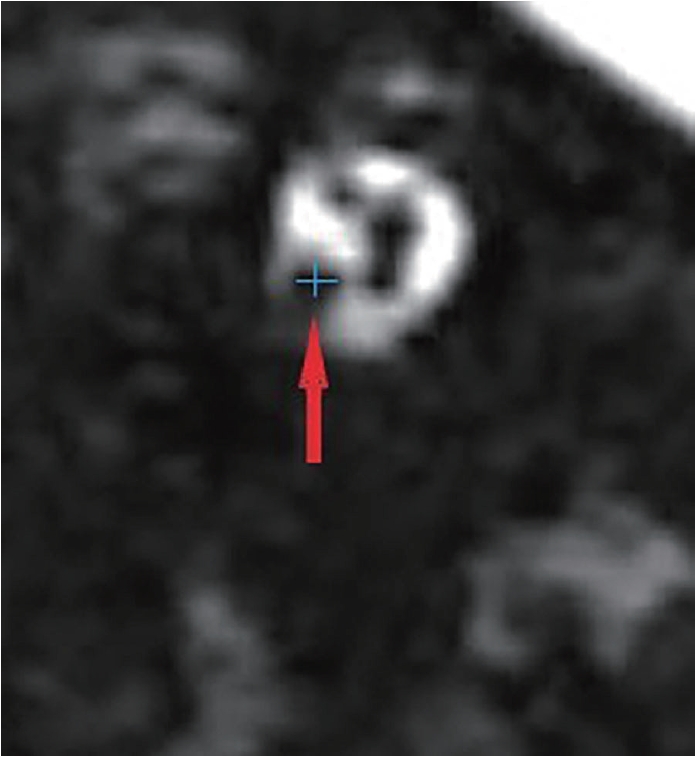
Table 1.
| Control group (n=60) |
Study group |
p-value | ||
|---|---|---|---|---|
| Unaffected side (n=125) | Affected side (n=125) | |||
| PTA (dB) | 20.8Âą6.9 | 20.7Âą6.7 | 57.6Âą27.3 | 0.94* |
| ïž0.01â | ||||
| SDS (%) | 96.6Âą3.8 | 96.8Âą3.6 | 62.3Âą35.9 | 0.93* |
| ïž0.01â | ||||
Table 2.
| Control group (n=120) |
Study group |
p-value | ||
|---|---|---|---|---|
| Unaffected side (n=125) | Affected side (n=125) | |||
| HD (mm) | 1 (0.7-1.5) | 1 (0.5-1.2) | 0.9 (0.4-1.2) | 0.47* |
| 0.02â | ||||
| 0.07⥠| ||||
| VD (mm) | 1.2 (0.9-1.7) | 1.2 (0.7-1.6) | 1.2 (0.5-1.6) | 0.76* |
| 0.04â | ||||
| 0.18⥠| ||||
| CSA (mm2) | 1 (0.3-1.8) | 0.9 (0.3-1.5) | 0.8 (0.2-1.4) | 0.51* |
| ïž0.01â | ||||
| 0.02⥠| ||||
Table 3.
|
General linear model |
|||
|---|---|---|---|
| F | Partial eta squared | p-value | |
| CSA (mm2) | 1.799 | 0.010 | 0.17 |
| HD (mm) | 0.942 | 0.005 | 0.39 |
| VD (mm) | 0.829 | 0.005 | 0.44 |
Table 4.
Data are presented as n (%). Complete recovery: all 5 frequencies of final audiogram are 20 dB or less or improvement to the same degree of hearing as in the unaffected ear; Marked improvement: PTA improvement ïž30 dB; Slight improvement: 10 dBïž PTA improvement ïž30 dB; No change: PTA improvement ïž10 dB. PTA, pure tone average
REFERENCES
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 4,131 View
- 231 Download
-
Clinical Aspects of Recurrent Idiopathic Sudden Sensorineural Hearing Loss2009 ;13(2)
Therapeutic Effect of Steroid on Sudden Sensorineural Hearing Loss2009 ;13(1)






