Introduction
Acute low-tone sensorineural hearing loss (ALHL) without vertigo is a different inner ear disease from conventional sudden idiopathic sensorineural hearing loss (SSHL). It is more common in females in their 40s. Most patients have accompanying low-pitched tinnitus, aural fullness, and may experience a light dizzy sensation (not true vertigo) [
1,
2]. In addition, frequent spontaneous recovery and recurrence occurs and there is a high risk of progression into MeniereŌĆÖs disease (MD) [
3].
The most used audiometric criteria for definite ALHL that was established by the Research Committee of the Ministry of Health, Labour and Welfare of Japan in 2011. It is as follows: 1) sum of hearing thresholds at 125, 250, and 500 Hz of Ōēź70 dB and 2) sum of hearing thresholds at 2,000, 4,000, and 8,000 Hz of Ōēż60 dB. Cases that meet the audiometric criteria 1) with the same hearing levels at 2,000, 4,000, and 8,000 Hz in the contralateral ear are defined as probable ALHL [
2]. ALHL is usually treated with combinations of steroids and diuretics compared with treatment for SSHL of oral steroids [
2]. ALHL is assumed to be caused by an autoimmune response of endolymphatic sac and/or cochlear-specific endolymphatic hydrops [
1,
4,
5]. Initial management is vital because the outcome after initial treatment of ALHL correlates with long-term results [
6]. However, optimal treatment remains unknown, such as the optimal concentration of initial daily steroids or most effective type of diuretics, or the efficacy of intratympanic steroid (ITS) injection.
In this study, we aimed to analyze the treatment pattern and outcome following different initial management to confirm the best treatment regimen.
Subjects and Methods
We retrospectively analyzed the medical records of 106 patients who visited an otology clinic at a Eulji University Hospital complaining of acute low-tone hearing loss from March 2013 to June 2019.
ALHL was defined by the following criteria: 1) a sensorineural hearing loss at low frequencies (125, 250, and 500 Hz), a sum of pure-tone thresholds at low frequencies of Ōēź70 dB and 2) a Ōēż60 dB sum of pure-tone thresholds at high frequencies (2, 4, and 8 kHz). 2) Intact tympanic membranes and 3) durations from symptom onset to treatment Ōēż14 days. The following conditions were excluded: 1) previous history of cochlear symptoms such as tinnitus, aural fullness and 2) nystagmus observed at initial visit or accompanying vertigo.
Hearing thresholds of 125, 250, 500, 1,000, 2,000, 3,000, 4,000, and 8,000 Hz were obtained, and the results of puretone audiometry performed at initial visit, 1 and 4 weeks after the initial visit were documented. The Institutional Review Board of the Eulji University Hospital approved this retrospective cohort study and waived the need to obtain written informed consent because of its retrospective nature (IRB number: 2020-12-002).
The study population comprised of 13 males and 36 females aged from 15-62 years, with a mean age of 41.96 years [standard deviation (SD): 12.56 years]. Mean duration from onset to treatment was 4.27 days (SD: 3.13 days, range: 0-14 days). For accompanying symptoms, aural fullness was the most frequent (91.8%, n=45), followed by tinnitus (55.1%, n=27) and transient dizzy sensation (28.6%, n=14). The affected side was the right in 17 (34.7%) and left in 32 (65.3%) patients. For accompanying diseases, three patients had diabetes and four had hypertension.
The treatment regimen was chosen based on the preference and/or clinical experience of doctors. Basic concepts for prescribing steroids were as follows: most patients in whom steroids were not administered at local clinic were initially treated with low-dose steroids [Ōēż30 mg of oral prednisolone (PD)]. If patients visited the local clinic complaining of persistent low-tone hearing loss, 60 mg of oral PD was initially prescribed.
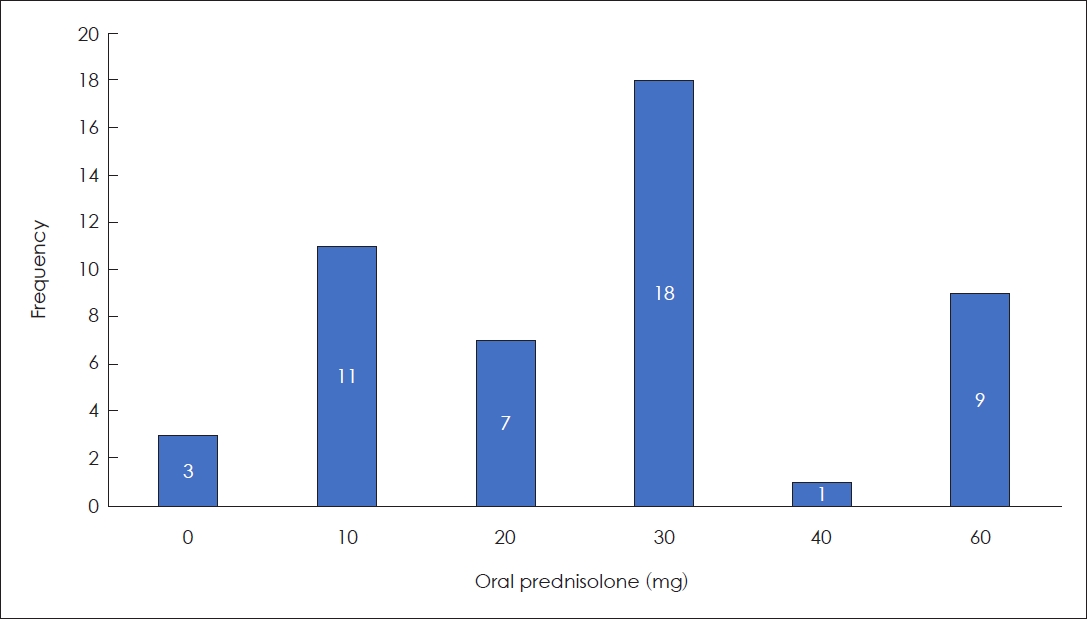
Oral PD was prescribed to 93.9% of patients (n=46), with mean initial daily dosage of 27.96 mg (SD: 18.141 mg, range: 10-60 mg, median: 30.00 mg) (
Fig. 1). PD was initially prescribed twice daily for 7 days if the initial daily dose did not exceed 30 mg. If the initial daily dose of PD was Ōēź30 mg, the same dose of initial PD was administered once a day for 4 days and then tapered 10 mg every 2 days.
Forty-five patients (91.8%) were treated with diuretics. Of these, 34 patients were treated with 50 mg of oral hydrochlorothiazide (75.6%) for 4 weeks and six patients (13.3%) with 70% 90 mL per day of isosorbide solution for 4 weeks and five patients (11.1%) were treated with 50 mg of oral spironolactone. For intratympanic dexamethasone injection, 18 patients (36.7%) received four concomitant intratympanic dexamethasone injections in 2 weeks. Two patients were treated with ITSs plus diuretics.
Complete recovery (CR) was defined if the mean hearing threshold of lower three frequencies at 125, 250, and 500 Hz was Ōēż20 dB.
To confirm which treatment modalities are effective, we performed a retrograde conditional logistic regression analysis of covariates that differed significantly by treatment outcome. This included use and types of diuretics, steroid dosage, and whether ITS was performed. All analyses were performed with the aid of IBM SPSS Statistics for Macintosh ver. 27.0 (IBM Corp., Armonk, NY, USA). p-values of <0.05 were considered statistically significant.
Results
Patient characteristics were summarized as
Table 1. The mean hearing threshold was calculated from 500, 1,000, 2,000, and 3,000 Hz at initial visit was 15.995 dB (SD: 6.649). Mean hearing thresholds at 125, 250, and 500 Hz and 2,000, 4,000, and 8,000 Hz were 33.129 dB (SD: 7.800 dB) and 11.259 dB (SD: 5.177 dB), respectively.
Forty-one patients (83.7%) showed CR at 2 weeks after initial visit and 43 patients (87.8%) showed a CR at 1 month later. Difference of CR at 2 week in accordance with initial treatment regimen is shown as
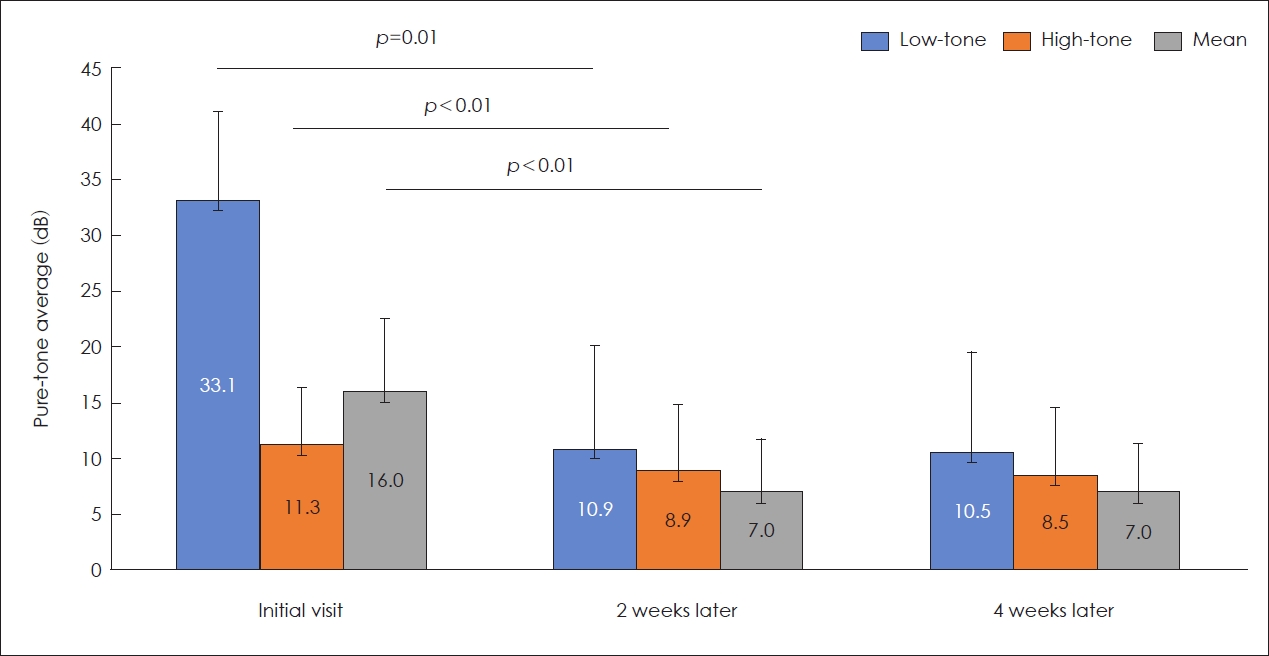
Fig. 2. For hearing thresholds, low-tone, high-tone, and mean hearing thresholds at 2 week improved significantly compared to those evaluated at initial visit (
p<0.1) (
Fig. 3). However, no significant differences were observed between hearing thresholds at 2 and 4 weeks.
Chi-square analysis was revealed that sex, affected side, accompanying symptoms, such as tinnitus, aural fullness, transient dizzy sensation, and accompanying diseases, including diabetes and hypertension did not differ according to recovery at 2 and 4 weeks (p>0.05).
With regard to treatment modalities, whether diuretics were used or what kind of diuretics were applied, used initial daily dose of oral steroids, ITS were not different according to CR at 2 and 4 weeks (p>0.05).
A retrograde conditional regression analysis with variables including initial steroid dosage, diuretics use and type, ITS, and dizzy symptoms was performed to confirm the prognostic factors for treatment outcome. CR at 2 weeks was associated with diuretic use [EXP(B)=10.309, 95% confidence interval (CI)=1.007-100]. An initial daily low-dose steroid use [EXP(B)=1.042, 95% CI=0.997-1.092, p=0.066] was marginally significant. Type of diuretics and ITS injection were not significant factors. For CR at 4 weeks, better recovery was associated with diuretic use [EXP(B)=25.641, 95% CI=1.121-90.909, p=0.039]; however, other treatment regimens did not affect final results.
Discussion
In this study, we found that use of diuretics was significantly associated with better treatment outcomes at 2 weeks and 1 month later irrespective of type. Recent MD guideline recommends that diuretics may be offered to patients to reduce or prevent symptoms and disease attacks [
7]. A systematic review on the use of diuretics for MD reported that vertigo was controlled and hearing improvements were achieved in 79% and 42.1% patients, respectively [
8].
Progression from ALHL to MD is a common; one study has reported that 27% of ALHL patients develop fluctuating hearing loss and 11% were finally diagnosed as classic MD [
5]. Accompanying tinnitus and recurrent episodes of ALHL are contributing factors for developing MD from ALHL [
9]. Similarly, 15.2% of ALHL patients have recurrence within a 1 year after initial outbreak and outcome after initial treatment is correlated with long-term results [
6].
We found that the type of diuretics administered did not affect treatment outcome. Our systematic review showed that the most commonly used diuretics for treatment of ALHL were thiazide diuretics (
Table 2). The most common complications of thiazide include dose-dependent metabolic imbalance such as hypokalemia, hypomagnesemia, hyperuricemia, decreased urinary calcium excretion, and glucose intolerance [
10]. However, these can be minimized by using lower dose [
10]. An oral osmotic diuretic, isosorbide solution, is an alternative. It is well tolerated for most of patients with MD with only a few experiencing headaches or nausea after medication [
11]. We hypothesize that changing the type of diuretics is a valid option if complications occur after initial diuretic administration. It is not a priority to change diuretic for better treatment outcomes because type of diuretics was not chosen in our regression model. However, an additional study with more larger sample size is necessary to confirm this.
We found that ALHL patients who were responsive to low-dose steroids tended to have better recovery at 2 weeks after initial treatment, though this was marginally significant. In a meta-analysis, no significant difference in recovery was observed between steroid treatment and diuretic treatment, implying that both are equally effective [
12].
In general, there is a belief that a higher dose of steroids may be more effective than a lower dose or where there is no improvement after initial treatment. Consistent with this, an early study dealing with ALHL reported that some patients who did not respond to an initial 60 mg intravenous and oral PD recovered after 200 mg intravenous PD, advocating use of higher dose steroids in ALHL [
13]. Similarly, corticosteroids can be effective at high doses within 7 days if initial treatment if the low doses fails to reduce symptoms [
14].
Interestingly, we found a tendency for better outcome at 2 weeks in patients who were treated initially with low-dose steroids. Therefore, low-dose steroids were sufficient for patients who are responsive to steroid treatment. Considering the mean or median value of steroid doses, an initial daily dose under 30 mg can be defined as a low dose. Due to the characteristics of the university referral hospital and retrospective nature of this study, patients who were less responsive to low-dose steroid treatment at the local clinic might be commonly treated with high-dose steroids in out clinic and they might be also less responsive to high-dose steroids.
A recent prospective study reported that the treatment effect of oral steroids alone (starting with 60 mg of oral PD) was significantly better than ITS as an initial treatment or oral steroids plus concurrent ITS [
15]. In fact, their inclusion criteria were somewhat different from other ALHL studies; therefore, their result was more closely related to idiopathic sudden hearing loss itself because they defined low-frequency hearing loss as 20 dB or more of hearing loss at 250, 500, and 1,000 Hz (1,000 Hz was included but 125 Hz was not included).
Similarly, a Japanese group compared definite ALHL and sudden low-tone loss that does not meet the criteria of ALHL [
16]. As a result, poor recovery and a tendency for more common recurrence was observed at 1 year in the sudden low-tone loss group; however, it was statistically insignificant. In their study, they also analyzed progression into MD with electrocochleography and electronystagmography [
16]. As a result, they argued that the current widely used inclusion criteria for ALHL may need to be complemented by additional electronystagmography and electrocochleography.
It was previously reported that ITS yielded a CR in most patients with ALHL [
17]. Partially consistent with our finding, a comparative study with a larger sample size reported no difference in treatment outcome between low dose steroids, high dose steroids, low dose steroids plus diuretics, and ITS plus diuretics [
18]. In contrast, ITS as a salvage treatment after 2 weeks of combined steroid-diuretic treatment yields better outcome than observation or continuous diuretics treatment alone for 1-year follow-up, though final treatment outcome after 5 years was not different, irrespective of treatment strategies [
19]. We did not find any beneficial effect of concurrent use of ITS; however, there is some scope to adopt ITS as a salvage treatment.
A Korean group has compared steroid treatment alone with combination treatments [
3]. Both treatment regimens improved hearing; however, there was a greater tendency for improvement in combination treatment but this was statistically insignificant.
Aside from treatment regimens, many prognostic factors have been revealed. Younger age, low-grade severity of initial hearing loss, early treatment, and female are good prognostic factors [
20]. On the other hand, accompanying tinnitus, occurrence of vertigo, bilateral involvement, longer symptom duration, and 1 kHz involvement are poor prognostic factors [
18,
21,
22].
Of the patients who showed final CR, two patients (a 60-year-old female and a 54-year-old male patient) showed a delayed recovery compared to other patients. They were treated with high-dose steroids plus hydrochlorothiazide and low-dose steroids plus hydrochlorothiazide and concomitant ITS, respectively. Their mean low-tone hearing thresholds at initial assessment were 30 and 60 dB and improved to 25 and 21.67 dB at 2 week, respectively. Therefore, we assumed that the individual difference in response to treatment might account for the delayed recovery more than expected, and more severe impairment may need more time for recovery.
Magnetic resonance imaging (MRI) has been investigated to reveal the pathogenesis of ALHL. In a 3T three-dimensional fluid attenuated inversion recovery study, cochlear endolymphatic hydrops was observed in 88.9% of ALHL patients who met the criteria of definite ATHL. Interestingly, vestibular endolymphatic hydrops were also found in these patients, though they had no vertigo, but some of them (36%) had subtle dizzy symptoms. These findings were similar to MD [
23]. These findings showed an increased volume of endolymphatic space/total fluid space in the vestibule in the recurrence group. Increased endolymphatic volume in the cochlea was observed in no cure group, suggesting that MRI can predict the prognosis of ALHL [
24].
Vestibular evoked myogenic potentials (VEMPs) have been used to predict the progression into MD or treatment outcome. Higher abnormal VEMPs suggestive of saccular hydrops are observed in MD compared with ALHL [
25]. Combining cVEMP and galvanic stimulation can predict final recovery more precisely by revealing the extent of saccular dysfunction [
26].
Taken together, our findings should be cautiously interpreted. We assume that patients who are unresponsive to low-dose steroids tend to have no or little response to higher steroids. Different from general sudden idiopathic hearing loss, initial low-dose steroids can be considered as a first-line treatment for ALHL patients in an out-patient clinic instead of high-dose steroid use. Diuretics should be used from the beginning of treatment. ITS as a salvage treatment may be considered.
Table 2 summarizes the characteristics of recent ALHL studies focusing on treatment outcome from 2002 to 2020 [
1,
3,
6,
13,
14,
15,
18,
19,
22,
27,
28]. Enrollment design, initial daily steroid dose, diuretics usage, ITS protocol, criteria for recovery varied widely from studies. For appropriate comparison, these should be unified first and then a prospective randomized controlled trial should be performed to determine the optimal treatment regimen.
In conclusion, combination of low-dose steroids under 30 mg of PD plus diuretics was sufficient for the first line treatment for ALHL. High-dose steroids and salvage ITS can be used a second choice treatment; however, the predictable outcome cannot be confirmed. Clinicians should counsel patients on these negative predictable results before the initiation of salvage treatment.