|
 |
- Search
| J Audiol Otol > Volume 16(3); 2012 > Article |
Abstract
Background and Objectives
The aim of this study is to determine whether the hyperproliferative and hyperkeratotic characters of cholesteatoma are associated with differentiation of keratinocytes in cholesteatoma by examining the localization of marker proteins, such as involucrin, filaggrin, and cytokeratins.
Materials and Methods
Immunohistochemical study was carried out in 30 cholesteatoma tissues and 10 retroauricular skins to examine the expression of involucrin, filaggrin, cytokeratin 4, 10 and 16. The staining results were graded as negative, weakly positive (<10%), moderately positive (10-70%), and strongly positive (>70%).
Results
Involucrin was strongly expressed in upper spinous, granular, and corneal layer of cholesteatoma. Filaggrin was strongly expressed in granular and corneal layer of cholesteatoma. Cytokeratin 4 was expressed in basal layer of retroauricular skin, but occasionally expressed in suprabasal layer of cholesteatoma. Cytokeratin 10 was homogenously expressed in all suprabasal layer of retroauricular skin, whereas pattern of shift to surface layer was showed in cholesteatoma. Cytokeratin 16 was moderately expressed at suprabasal layer in cholesteatoma.
Middle ear cholesteatoma is defined pathologically as the abnormal existence of keratinized squamous epithelium in the middle ear cavity, leading to bone destruction of middle ear structures and their surroundings, and is characterized by unusual hyperkeratosis and hyperproliferation. Epithelial homeostasis in normal skin is maintained by a delicate balance between proliferation and terminal differentiation rate.1) On the other hand, it has been proposed that the development of human cholesteatoma is due to altered control of cellular proliferation, which tilts the balance toward the aggressive, invasive growth of squamous epithelium into middle ear. Histopathologically, basal cell hyperplasia, appearance of suprabasal cells with new proliferation capacity are observed in cholesteatoma, resulting in disruption of epidermal homeostasis between proliferation and terminal differentiation rate.2-4)
Involucrin is precursor of human cornified envelope and can be a marker for the intermediate stage of squamous differentiation.5,6) Filaggrin is a major component of keratohyalin, and is concerned with differentiating of granular and corneal layer.7,8) Cytokeratins (CK) are known to be insoluble proteins that form the intermediate filaments of mammalian cells, and have been introduced as markers of cellular proliferation.9,10)
The aim of this immunohistochemical study is to determine whether the hyperproliferative and hyperkeratotic characters of cholesteatoma are associated with differentiation of keratinocytes in cholesteatoma in comparison to retroauricular skin (RAS), by examining the localization of marker proteins, such as involucrin, filaggrin, and CKs, which is typically expressed in different steps of squamous epithelial differentiation.
We obtained cholesteatoma tissue from 30 subjects during surgery as study group, and normal RAS tissue from 10 subjects as control group.
Immunohistochemical staining was performed to evaluate the expression of involucrin, filaggrin, CK 4, CK 10 and CK 16. The tissues were fixed in 10% buffered neutral formalin solution and embedded in paraffin. For immunohistochemical staining, 4 ┬Ąm-paraffin embedded tissue sections were prepared on Leica Plus slide (Leica Biosystems Richmond, Inc., Richmond, IL, USA). The tissue sections were deparaffinized 3 times in dewax solution (Leica Biosystems Newcastle Ltd., Newcastle, UK) for 1 minute at 72Ōäā, and then treated 4 times in 100% ethyl alcohol 1 minute respectively, prior to rehydration with distilled water. Antigen retrieval was performed by heating the slides for 15 minute in Epitope Retrieval Solution 1, 2 (Leica Biosystems Newcastle Ltd., Newcastle, UK) and by cooling for 12 minutes. The slides were then treated with 3% H2O2 in Bond Polymer Refine Kit (Leica Biosystems Newcastle Ltd., Newcastle, UK) 10 minutes quench endogenous peroxidase activity and washed 3 times in Bond Wash Solution (Leica Biosystems Newcastle Ltd., Newcastle, UK) for 1 minute. After that, sections were incubated for 15 minutes with 1 : 100 dilution of monoclonal antibody to CK 4 (Leica Biosystems Newcastle Ltd., Newcastle, UK), CK 10 (Leica Biosystems Newcastle Ltd., Newcastle, UK), Filaggrin (Leica Biosystems Newcastle Ltd., Newcastle, UK), 1 : 50 dilution of monoclonal antibody to CK 16 (Leica Biosystems Newcastle Ltd., Newcastle, UK) and 1 : 200 dilution of monoclonal antibody to Involucrin (Leica Biosystems Newcastle Ltd., Newcastle, UK). After washing the sections 3 times with Bond Wash Solution for 1 minute, we incubated the sections for 8 minute with the Post Primary in Bond Polymer Refine Kit. After wash with Bond Wash solution and for an additional 8 minutes with Polymer in Bond Polymer Refine Kit. After washing with Bond Wash Solution, 3,3'-diaminobenzidine tetrahydrochloride (DAB) in Bond Polymer Refine Kit for 10 minutes was used to visualize the peroxidase activity and hematoxylin was used to counterstain. All operate with Bond-maX auto immuno stainer (Leica Biosystems Melbourne Pty Ltd., VIC, Australia). The sections were mounted with Canada balsam.
All specimens were independently analyzed by two independent observers who were blinded to the specimen information. Both the basal, suprabasal (upper spinous, lower spinous and granular) and corneal layers of the epitheliums of normal RAS and cholesteatoma were analyzed for positive staining. The positive-staining rates were determined in the areas where the full thickness of the epidermis could be clearly seen, and we designated cells that stained brown in the cytoplasm as positive. The results for the specimen training were graded as negative (-), weakly positive (+) if the percentage of positive cells was under 10%, moderately positive (++) if 10-70%, and strongly positive (+++) if over 70%. After the identification of well-stained areas under a light microscope at 100-fold magnification, we calculated the average percentage of three different spots at 400-fold magnification.
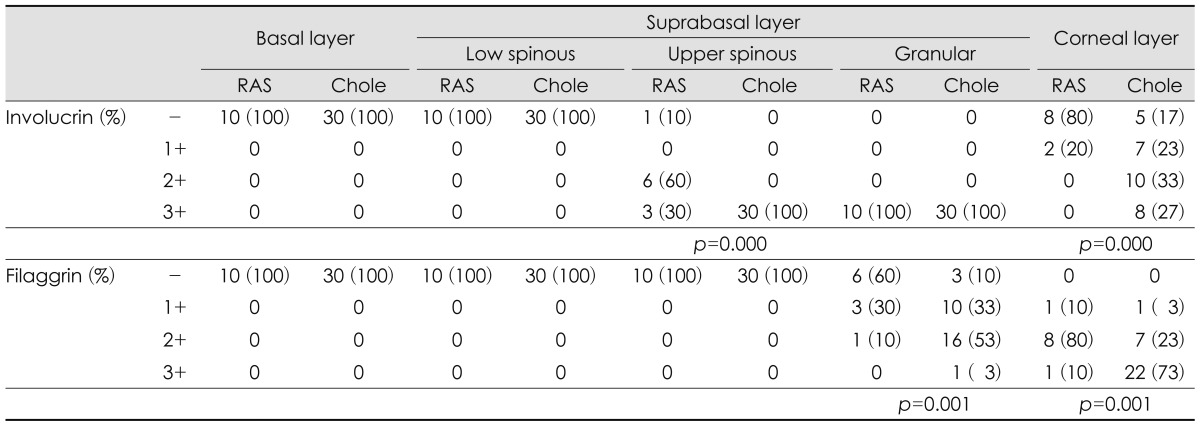
Involucrin was expressed in upper spinous, granular and corneal layers of RAS and cholesteatoma. In upper spinous layer, involucrin expression was moderately positive in most of RAS but strongly positive in all cholesteatomas (p=0.000). In granular layer, involucrin expression is strong in all RAS and cholesteatoma. In corneal layer, cholesteatoma showed moderately or strongly positive expression of involucrin, compared with negative expression in most of RAS (p=0.000)(Table 1)(Fig. 1).
Filaggrin was expressed in granular and corneal layers of RAS and choelsteatoma. In granular layer, filaggrin expression was negative or weakly positive in most of RAS, but weakly or moderately positive in cholesteatoma (p=0.001). In corneal layer, filagglin expression was strongly positive in most of cholesteatoma but not in RAS (p=0.001)(Table 1)(Fig. 1).
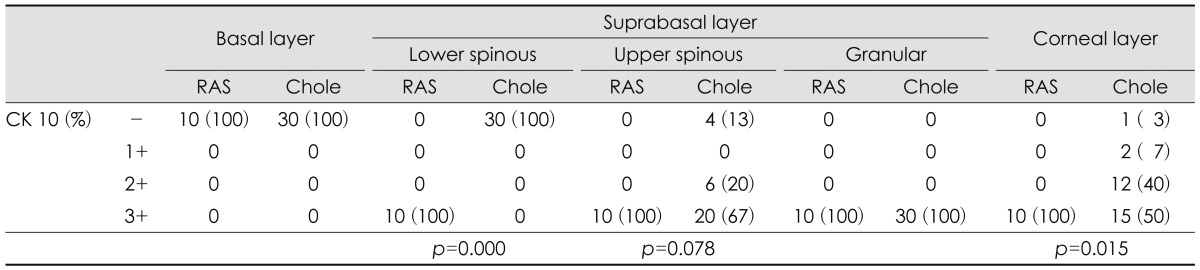
CK 4 was expressed in basal layer of RAS with but not in cholesteatoma (p=0.000). In suprabasal layer, there was no difference between RAS and cholesteatoma (p=0.542). Both RAS and cholesteatoma were not positive to CK 4 in corneal layer (Table 2)(Fig. 2). CK 16 was expressed in only basal layer of RAS. Half of cholesteatoma showed moderately or strongly positive expression at suprabasal layer (p=0.006)(Table 2)(Fig. 2).
CK 10 was homogenously strong-positive in all suprabasal and corneal layers of RAS. While cholesteatoma showed negative expression in lower spinous layer (p=0.000), moderately to strongly positive in upper spinous layer (p=0.078), and strongly positive in granular layer. In corneal layer, CK 10 expression was weaker in most of the cholesteatoma than RAS (p=0.015) (Table 3)(Fig. 2).
Middle ear cholesteatoma is defined pathologically as the abnormal existence of keratinized squamous epithelium in the middle ear. As soon as the epithelium begins to hyperkeratinize, the destructive behavior is triggered. Regardless of their underlying many pathogenesis, all cholesteatomas share similar pathology and properties. Their common clinical hallmarks are invasion, migration, hyperproliferation, altered differentiation, aggressiveness and recidivism.11) And modern technologies using immuohistochiemistry, in situ hybridization, polymerase chain reaction, microarrays et al were applied to improve understating of the biologic properties of cholesteatoma.11) Unlike normal skin, which has the karatinocytes proliferation capacity only in the basal cell layer of the epidermis, many studies for proliferative activity of cholesteatoma reported basal cell hyperplasia and appearance of suprabasal cells with new proliferation capacity. Normal keratinocytes that have lost the ability to divide move toward the suprabasal cell layers and terminally differentiated keratinocytes are anucleate and contain abundant cross-linked keratin filaments forming a cornified protein envelop. Epidermal homeostasis is maintained by a delicate balance between proliferation and terminal differentiation rate.1) In cholesteatoma, Ki-67, a marker of cell proliferation, positive cells have been significantly observed in the suprabasal layer12) and proliferating cell nuclear antigen positive cells have been detected in the suprabasal and parabasal layer as well as in the basal layer of cholesteatoam epithelium.13) Epidermal growth factor receptor also highly expressed in basal and suprabasal keratinocytes in cholesteatoma.14)
CKs used in this study are intermediated filaments proteins that are exclusively present in epithelial cells. The expression of different types of CKs (numbered 1-20) varies with the type of epithelium and the stage of differentiation.9) According to the study of Kakoi, et al.,15) the expression of CKs 6, 16, and 19 in cholesteatoma epithelium showed hyperproliferative pattern. Patterns of the terminal differentiation of CKs 1, 5, 10 and 14 in cholesteatoma were basically the same as those in skin.
According to Kim, et al.,16) CK expression correspond well with the state of keratinocyte proliferation, migration and differentiation. The result of their study using an animal model supports the concept that the normal keratinizing epithelium of the tympanic membrane undergoes certain changes as it forms a cholesteatoma in the external canal and middle ear. An increased expression of CK 5/6 and CK 13/16 is found in the suprabasal epithelial layer of the external auditory canal can be an evidence that cholesteatoma shows increased keratinocyte proliferation.
CKs used in this study were CK 4, 10 and 16. CK 4 stains basal layers of normal epidermis and appears in less differentiated keratinocytes. CK 4 is marker for non-keratinizing epithelia and indicates an altered differentiation and migration of keratinocytes.10) CK 10 is normally localized in the suprabasal layer of the epidermis and it's expression corresponded to the extent of differentiation within keratinocytes, so it can be a marker for differentiatioin.10,17) CK 16, a marker of proliferation, is partially expressed in basal layer of normal epidermis, but expressed in suprabasal layer in many epidermal proliferative diseases.16,18)
Our study showed CK 4 was expressed in basal layer of all RAS. But in cholestetoma, there was no expression in basal layer while 33.3% expression in suprabasal layer. It means altered differentiation of cholesteatoma. CK 10 was strongly and homogenously expressed in all suprabasal and corneal layers of normal RAS. On the other hand, in cholesteatoma CK expression was getting stronger from lower to upper layer. This pattern of 'shifting to surface layer' presumed immature keratinocyte differentiation, and also presumed different velocity of differentiation, namely more faster in upper layer, compared with balanced differentiation of normal skin. Some other studies support our results,19,20) but Kim, et al.16) reports CK 10 expression may be affected by the extent of cholesteatoma and the severity of disease.
CK 16 was occasionally expressed in only basal layer of normal skin, but strong expressed suprabasal layer in cholesteatoma. This prominent changes suggested hyperproliferative process of cholesteatoma.
Hyperkeratosis was resulted from not only the hyperproliferation of basal and suprabasal layer, but also early differentiation of suprabasal layer in process of terminal differentiation from basal layer to corneal layer. The useful marker of this terminal differentiation was involucrin and filaggrin.
Involucrin is the major protein component of the cross-linked envelope synthesized by maturing cells of human stratified squamous epithelia with a molecular weight of 140 kD. It is precursor of human cornified envelope. It is detectable in granular and upper spinous layer in normal skin, but not in both basal and corneal layer, so serves as a marker for the intermediate stage of squamous differentiation.5,6,21) Stammberger, et al.21) reported that in cholesteatoma, involucrin was localized in the suprabasal cells, and particularly in spinous cell, and it appeared earlier than in ear canal skin. A larger amount of involucrin was present in cholesteatoma (60%) than in external ear canal skin (29%) and in normal skin (25%) in Chao's study.22) Park, et al.23) presented that involucrin was highly expressed in deep meatal skin as well as cholesteatoma than RAS, which may support the migration theory of cholesteatoma pathogenesis.
In our study, similar expression was noted at granular layer of retroauricular skin and cholesteatoma. But in upper spinous layer involucrin was strongly expressed in all cholesteatomas than normal skin. In corneal layer, cholesteatoma showed highly positive expression of involucrin, compared with negative expression in most of RAS. This results presumed that hyperdifferentiation and hyperkeratosis might be resulted from early differentiation of upper spinous layer.
Filaggrin, a histidine-rich proteins, is a component of kertohyalin that is formed in differentiating cells. During formation of cornified cells, keratohyalins are dispersed in the interfilatmentous spaces. The basic filaggrins aggregate keratin filaments into large insoluble macrofibrils, conferring flexibility to the stratum corneum. Thus filaggrin can be a marker of epidermal differentiation.7,8) Commonly, filaggrin was expressed in corneal layer of normal skin, whereas in cholesteatoma, it was expressed more in corneal and granular layer.7,21,23) Our immunohistochemical study also got a similar results. Filaggrin overexpression in granular and corneal layer presumed that early differentiation may induce the typical hyperkeratosis of cholesteatoma.
Based on these results, it can be suggested that early differentiation of suprabasal layer, especially upper spinous and granular layers in cholesteatoma, may lead to hyperdifferentiation and hyperkeratosis. Different expression of CKs, compared with RAS, possibly indicates the altered differentiation and hyperproliferation of cholesteatoma.
Overexpression of involucrin and fillagrin presumed that early differentiation of suprabasal layer, especially upper spinous and granular layers in cholesteatoma, may lead to hyperdifferentiation and hyperkeratosis. Suprabasal expression of CK 4 and 16 and pattern of shifting to surface layer in CK 10, compared with RAS, possibly indicate the altered differentiation and hyperproliferation of cholesteatoma.
References
1. Watt FM. Proliferation and terminal differentiation of human epidermal keratinocytes in culture. Biochem Soc Trans 1988;16:666ŌĆō668. PMID: 2466709.


2. Park K, Chun YM, Park HJ. Cytokeratin expressions of induced cholesteatoma in gerbill. Korean J Otolaryngol-Head Neck Surg 1996;39:747ŌĆō753.
3. Chao WY, Huang CC. An immunocytochemical study of cytokeratin expression in human middle ear cholesteatoma. Arch Otorhinolaryngol 1989;246:37ŌĆō42. PMID: 2472127.


4. Buj├Ła J, Schilling V, Holly A, Stammberger M, Kastenbauer E. Hyperproliferation-associated keratin expression in human middle ear cholesteatoma. Acta Otolaryngol 1993;113:364ŌĆō368. PMID: 7685977.


5. Murphy GF, Flynn TC, Rice RH, Pinkus GS. Involucrin expression in normal and neoplastic human skin: a marker for keratinocyte differentiation. J Invest Dermatol 1984;82:453ŌĆō457. PMID: 6392430.


6. Rice RH, Green H. Presence in human epidermal cells of a soluble protein precursor of the cross-linked envelope: activation of the cross-linking by calcium ions. Cell 1979;18:681ŌĆō694. PMID: 42494.


7. Chao WY, Huang CC. Localization of filaggrin in human middle ear cholesteatoma. Acta Otolaryngol 1989;107:249ŌĆō253. PMID: 2648749.


8. Harding CR, Scott IR. Histidine-rich proteins (filaggrins): structural and functional heterogeneity during epidermal differentiation. J Mol Biol 1983;170:651ŌĆō673. PMID: 6195345.


9. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982;31:11ŌĆō24. PMID: 6186379.


10. Olszewska E, Sudhoff H. Comparative cytokeratin distribution patterns in cholesteatoma epithelium. Histol Histopathol 2007;22:37ŌĆō42. PMID: 17128409.

11. Olszewska E, Wagner M, Bernal-Sprekelsen M, Ebmeyer J, Dazert S, Hildmann H, et al. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol 2004;261:6ŌĆō24. PMID: 12835944.


12. Bujia J, Holly A, Sudhoff H, Antoli-Candela F, Tapia MG, Kastenbauer E. Identification of proliferating keratinocytes in middle ear cholesteatoma using the monoclonal antibody Ki-67. ORL J Otorhinolaryngol Relat Spec 1996;58:23ŌĆō26. PMID: 8718533.


13. Park K, Park HJ, Chun YM. Immunohistochemical study on proliferative activity of experimental cholesteatoma. Eur Arch Otorhinolaryngol 2001;258:101ŌĆō105. PMID: 11374247.


14. Buj├Ła J, Kim C, Holly A, Sudhoff H, Ostos P, Kastenbauer E. Epidermal growth factor receptor (EGF-R) in human middle ear cholesteatoma: an analysis of protein production and gene expression. Am J Otol 1996;17:203ŌĆō206. PMID: 8723947.

15. Kakoi H, Tamagawa Y, Kitamura K, Anniko M, Hiraide F, Kitajima Y. Cytokeratin expression patterns by one- and two-dimensional electrophoresis in pars flaccida cholesteatoma and pars tensa cholesteatoma. Acta Otolaryngol 1995;115:804ŌĆō810. PMID: 8749203.


16. Kim HJ, Tinling SP, Chole RA. Expression patterns of cytokeratins in cholesteatomas: evidence of increased migration and proliferation. J Korean Med Sci 2002;17:381ŌĆō388. PMID: 12068144.



17. Olszewska E, Lautermann J, Koc C, Schwaab M, Dazert S, Hildmann H, et al. Cytokeratin expression pattern in congenital and acquired pediatric cholesteatoma. Eur Arch Otorhinolaryngol 2005;262:731ŌĆō736. PMID: 15754169.


18. Weiss RA, Eichner R, Sun TT. Monoclonal antibody analysis of keratin expression in epidermal diseases: a 48- and 56-kdalton keratin as molecular markers for hyperproliferative keratinocytes. J Cell Biol 1984;98:1397ŌĆō1406. PMID: 6201492.



19. Shinkawa H, Hozawa J, Usami S, Saito S. Immunohistochemical study on cytokeratin and vimentin expression in congenital cholesteatoma. In: Nakano Y. editor. Cholesteatoma and Mastoid Surgery. 1993. Amsterdam: Kugler;p.155ŌĆō166.
20. Broekaert D, Coucke P, Leperque S, Ramaekers F, Van Muijen G, Boedts D, et al. Immunohistochemical analysis of the cytokeratin expression in middle ear cholesteatoma and related epithelial tissues. Ann Otol Rhinol Laryngol 1992;101:931ŌĆō938. PMID: 1280020.


21. Stammberger M, Buj├Ła J, Kastenbauer E. Alteration of epidermal differentiation in middle ear cholesteatoma. Am J Otol 1995;16:527ŌĆō531. PMID: 8588655.

22. Chao WY, Huang CC. Expression of involucrin in human middle ear cholesteatoma. Am J Otol 1989;10:385ŌĆō388. PMID: 2683803.

23. Park KH, Park HJ, Chun YM, Lee JS, Kim HJ. Immunohistochemical study for differentiation of human middle ear cholesteatoma. Korean J Otolaryngol-Head Neck Surg 1998;41:1521ŌĆō1526.
Fig.┬Ā1
Immunohistochemical demonstration of involucrin (A, B) and filagglin (C, D)(├Ś400). In cholesteatoma (B), involucrin shows stronger positive expression at upper spinous and corneal layers than in retroauricular skin (A). Filaggrin show much strongly positive staining at granular and corneal layers in cholesteatoma (D) than in retroauricular skin (C). Retroauricular skin (C) shows moderately positive staining to filagglin at corneal layer.
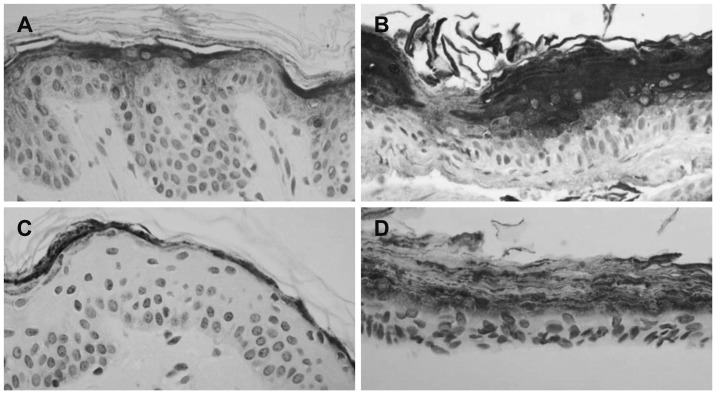
Fig.┬Ā2
Immunohistochemical demonstration of cytokeratin 4 (A, B),10 (C, D) and 16 (E, F) in retroauricular skin and cholesteatoma, respectively (├Ś400). Cytokeratin 4 is expressed at basal layer in retroauricular skin (A). On the other hand, cholesteatoma (B) shows moderately positive staining at suprabasal layer. Cytokeratin 10 shows homogenously strongly positive staining at all suprabasal and corneal layers in retroauricular skin (C) while cholesteatomas (D) show relatively weak staining at upper spinous layer. Cytokeratin 16 is expressed at basal layer in retroauricular skin (E) and cholesteatoma (F) shows moderately positive staining at suprabasal layer.

Table┬Ā1
Comparison of involucrin and filaggrin expression in retroauricular skins (n=10) and cholesteatomas (n=30)