Introduction
Chronic otitis media (COM) is the chief cause driving hearing impairment in children and young adults in developing countries like India [
1]. It commonly presents with mild chronic symptoms like ear discharge and hearing impairment. However, many times, it does present in advanced stages, having caused an extracranial or intracranial complication. Cholesteatomas with squamosal COM are particularly known for causing complications due to their inherent ability to erode bone, while mucosal COM rarely present with complications. A prolonged untreated disease was previously believed to be the main cause of complications. Even children with a short symptom history have been known to sustain these complications, suggesting that there might be inherent properties of the epithelium which render it aggressive. The reasons for this varied aggressiveness of cholesteatoma are poorly understood.
Cytokeratins are structural proteins found in the intracytoplasmic cytoskeleton of epithelial cells. They are abundantly expressed in squamous epithelia which are exposed to the environment, e.g., oral mucosa, ear canal skin, etc. All cytokeratins are not expressed simultaneously in a cell but are expressed in different stages of development during their course of terminal differentiation. Based on the stage of maturation of squamous epithelium, differential expression of various cytokeratins is seen across its layers [
2]. 34ße12 is a cocktail of high molecular weight keratins including cytokeratins (CK) 1, 5, 10, and 14. CK5 and CK14 are expressed in basal cells while CK1 and CK10 are expressed in the entire suprabasal compartment of complex stratified epithelia. It is normally expressed in all the layers of squamous epithelium and is a marker of mature stratified squamous epithelia [
3]. CK17 is normally expressed in the basal cells of complex epithelia and epithelial appendages such as nails, hair follicles, sebaceous glands, and eccrine sweat glands. It has been found to be aberrantly expressed in suprabasal keratinocytes of hyper-proliferative cutaneous disorders like psoriasis [
4]. CK13 is predominantly expressed in suprabasal cells of mature non-keratinizing stratified squamous epithelium, and its expression is suppressed in hyperproliferative disorders [
5]. Ki67 is a nuclear antigen that is expressed in proliferating cells and can be used as an immunohistochemical marker to assess increased proliferative activity of squamous epithelial cells, and hence to mark the nature of disease [
6].
In this study, we attempt to observe the cytokeratin expression among cholesteatoma patients as compared to diseasefree controls. We then categorize disease patterns clinico-radiologically and correlate their aggressiveness with patterns of immunoexpression of various keratins and Ki67 staining.
Subjects and Methods
Study duration and location
This prospective case-controlled study was conducted over four years (2017–2021) at our tertiary care center in North India. It was approved by the Institutional Ethics Committee (IEC-621/02.11.2018, RP-35/2018).
Study population
We recruited all consecutive patients of COM, squamosal type with cholesteatoma. All patients’ disease status was staged in accordance with the European Academy of Otology and Neurotology/Japanese Otological Society (EAONO/JOS) staging by three experts (AK1, RM, and YV), which divides the disease into four stages. Stage I refers to disease limited to the primary site (usually epitympanum), stage II refers to spread of disease to more than one site, stage III refers to an extracranial complication, and stage IV is assigned to an intracranial complication [
7]. The patients who did not agree to participate or were unfit for surgery were excluded.
Controls
The patients of COM, mucosal type, who were planned for tympanoplasty at our center were recruited as controls, after obtaining consent from the involved subjects.
Specimen collection
A diagnosis of COM, squamosal type was made using otoscope, and confirmed and staged with radiological examination (high resolution computed tomography [HRCT] of temporal bone) by two experts (AK1 and KS). All patients were planned for canal wall up/down mastoidectomy, depending on the surgical extent of disease. All the patients had their disease completely removed. The epithelium from the excised cholesteatoma specimen was taken. As controls, we took 1–2 mm piece of skin from the bony external auditory canal (EAC) of the control group while elevating the tympanomeatal flap. Both the case and the control specimens were preserved in 10% formalin and sent for routine histopathological examination and immunohistochemistry (IHC).
Immunohistochemistry
Specimens that included the full thickness of cholesteatoma matrix (keratinized squamous epithelium) were chosen for immunohistochemical staining. IHC was performed with a panel of primary monoclonal antibodies viz. 34βE12 (clone 34βE12; Thermo Fisher Scientific; 1:200 dilution), CK17 (clone E3; Thermo Fisher Scientific; prediluted), CK13 (clone KS-1A3; Thermo Fisher Scientific; prediluted), Ki67 (clone IHC067; GenomeMe; 1:200 dilution) using appropriate positive and negative controls. Five-micron thick sections were cut from the formalin-fixed and paraffin-embedded (FFPE) blocks onto poly-L-lysine coated slides. After deparaffinization and rehydration in descending grades of alcohol, the sections were brought to water. Antigen retrieval was performed by transferring the sections into 0.01 M citrate buffer, pH 6.0, previously heated in a microwave oven. After washing in Tris buffer (pH 7.6) and blocking with 3% H2O2 in methanol for 30 minutes at room temperature, the sections were incubated overnight at 4°C with the primary antibodies. Subsequently, the sections were treated with the polymer-labeled, HRP-conjugated secondary antibody detection system. Diaminobenzidine chromogen was put on the sections for 10 minutes. The sections were washed, counter-stained in hematoxylin, and then mounted. Cytoplasmic staining for 34βE12, CK17, and CK13, and nuclear labeling for Ki67 was considered positive. The staining patterns of immunohistochemical markers were evaluated based on the epithelial layers in which they were expressed.
Statistical analysis
Based on their predominant patterns of immunostaining, we divided the cases into 2–3 subgroups for ease of statistical analysis. These patterns of staining were then compared with the controls using Fisher exact test due to the small specimen size of controls. Comparison between different subgroups was also performed using Fisher exact test and chi-square test. Where multiple subgroups were analyzed together, Freeman-Halton extension of Fisher exact test was used. SPSS 27.0 (IBM Corp., Armonk, NY, USA) was used for performing statistical analysis. A p-value of <0.05 was considered significant.
RESULTS
We could recruit 52 patients into the study; however, 11 specimens did not show cholesteatoma matrix, i.e., squamous epithelium and IHC could not be performed. The remaining 41 specimens underwent IHC and were further analyzed. Out of 41 patients, 14 (34%) were female. The mean age was 25 years (range 4–68 years), the median age was 21 years. All 41 patients had ear discharge and hearing loss at the time of presentation; 39 patients (95%) had conductive hearing loss while two patients had profound hearing loss on the affected side. Six patients (14.6%) had dizziness and vertigo at the time of presentation out of which four patients (9.7%) were found to have lateral semicircular canal fistula.
Ten patients (24.4%) belonged to stage I, 27 patients (65.9%) belonged to stage II, four patients (9.7%) belonged to stage III, and no patient belonged to stage IV. All 41 patients underwent canal wall down mastoidectomy as the surgical treatment. The clinico-demographic details of the recruited subjects have been tabulated in
Table 1.
Results of Immunohistochemistry
The comparison of immunoexpression of the cytokeratins in specimens with their corresponding clinical stage is shown in
Table 2.
34ße12 staining
Thirty-three specimens showed 34ße12 staining. Of these, 31 (75.6%) specimens demonstrated 34ße12 immunoexpression across all the epithelial layers (
Fig. 1A), while two (4.8%) specimens showed 34ße12 staining in the superficial layer only. Eight (19.5%) specimens showed no staining with 34ße12 (
Fig. 1B), indicating a complete lack of mature squamous differentiation.
CK13 staining
CK13 staining was seen in 32 specimens. Of these, eight (19.5%) specimens demonstrated CK13 across all the layers of the epithelium (
Fig. 2A). Eleven (26%) specimens showed basal pattern of CK13 immunoexpression. Thirteen (31.7%) specimens expressed CK13 in basal and suprabasal epithelial cells. Nine (22%) specimens did not express CK13 (
Fig. 2B), indicating hyperproliferation. All these CK13-negative specimens expressed 34ße12 across all layers, consistent with squamous epithelium with increased proliferative activity. Conversely, six of the eight specimens that were negative for 34ße12 expressed CK13 across all layers, indicating a non-proliferative, non-squamous phenotype.
CK17 staining
CK17 staining was seen in 38 specimens. Of these, two (4.8%) specimens showed full thickness expression of CK17, 13 (31.7%) showed suprabasal staining (
Fig. 3A), 12 (29.2%) specimens showed suprabasal and superficial pattern of staining, and 10 (24.3%) specimens demonstrated expression of CK17 in superficial layers only (
Fig. 3B). Basal and superficial pattern of staining was seen in one (2.4%) specimen only. Three (7.2%) specimens did not show any expression of CK17.
Ki67 staining
Ki67 staining was restricted to the basal and suprabasal cells, i.e., ≤3 layers of epithelial cells in 28 (68%) specimens (
Fig. 4A). Nine (22%) specimens showed increased staining of Ki67 across >3 layers of epithelial cells (
Fig. 4B) indicative of increased proliferation. Ki67 staining could not be performed on 3 specimens due to exhaustion of tissue in the paraffin blocks.
Statistical analysis
For ease of statistical analysis, we made 2–3 subgroups for each IHC stain by clubbing multiple similar patterns of staining together.
1) For 34ße12, two subgroups were made: one where the specimens showed full-thickness expression and the other where specimens showed no expression of 34ße12.
2) For CK17, the specimens were divided into suprabasal group (which included suprabasal pattern, full-thickness pattern, and suprabasal+superficial pattern), superficial only group, and negative group which did not express CK17 at all.
3) For CK13, the specimens were divided as full-thickness expression group, patchy expression group (suprabasal, basal, or both), and negative staining group.
5) For Ki67 stain, we divided the patients as low proliferation (0–3 layers) and high proliferation groups (>3 layers).
Table 3 shows the subgroups and the corresponding number of specimens in each group against it.
Controls
IHC on all 11 control specimens from EAC skin showed 34ße12 staining in the entire thickness of the squamous epithelium. CK17 was completely negative. CK13 showed positivity in the basal layer of four specimens while the rest did not show any CK13 staining. All specimens showed low Ki67 labeling (up to 2 layers) in basal and suprabasal epithelial layers.
Statistical analysis
There was no difference in expression of 34ße12 between cases and controls (p=0.09). There was a significant difference in the staining patterns of CK17 (p<0.001), CK13 (p<0.03), and Ki67 (p<0.001) between cholesteatoma specimens and controls.
When stage-wise analysis was performed, there was no difference in expression of 34ße12 (
p=0.63), CK17 (
p=0.35), CK13 (
p=0.53), and Ki67 (
p=0.72) between different clinical stages (
Table 3). On comparing the cytokeratin expression patterns of the ten 34ße12-negative cases, eight demonstrated a full-thickness expression and two demonstrated patchy expression of CK13. Similarly, all eight specimens which belonged to CK13-negative group demonstrated a full-thickness expression of 34ße12. This pattern of “reciprocal” expression between the high-molecular weight and low-molecular weight cytokeratins was found to be statistically significant (
p<0.001). Although CK17 staining was seen to be increased in specimens with high Ki67, this finding was not statistically significant (
p=0.12). There were no other significant differences between the subgroups.
There was no correlation between the cytokeratin staining patterns and clinical variables like age, sex, duration of ear symptoms, the type (conductive vs. sensorineural) or severity of hearing loss present, and clinical stage.
DISCUSSION
This study demonstrates an increased expression of CK17, CK13, and Ki67 in cholesteatoma specimens when compared to normal bony EAC controls, and loss of expression of 34ße12 in a subset of cholesteatoma specimens. Hamed, et al. [
6] also found an increased expression of CK17 and Ki67 as compared to the skin controls in their study although their work did not demonstrate increased expression of CK13 in cholesteatoma specimens. Our previous work in this regard had also demonstrated a significantly increased expression of Ki67 in cholesteatoma specimens when compared to controls [
8]. Almost all previous studies have reported similar findings [
9–
13].
Sasaki and Huang [
5] in their study demonstrated increased expression of CK13 in the basal layer of cholesteatoma, while no expression was observed in meatal skin and tympanic membrane. We observed a range of staining patterns for CK13 in cholesteatoma specimens, right from no staining, staining restricted to basal and suprabasal layers, to full-thickness staining. Four of our control specimens also demonstrated CK13 staining in the basal layer. We observed a significant “reciprocal” pattern of staining between CK13 and 34ße12. Our findings lend support to the hypothesis of squamous metaplasia of middle ear mucosa leading to cholesteatoma in the cases where full-thickness expression of CK13 was observed and no expression of 34ße12 was seen. CK13 is an LMW keratin expressed in mucosal epithelia while 34ße12 is an HMW keratin expressed in complex stratified epithelium like squamous and urothelial mucosa. This corresponds to the squamous metaplasia theory by Sadé, which states that cholesteatoma occurs due to squamous metaplasia of primarily glandular epithelium of middle ear mucosa in setting of chronic inflammation like COM [
14].
We could observe no difference in the expression pattern of 34ße12 between the cases and the controls. Olszewska and Sudhoff [
3], however, reported contrary findings to our work as they found an increased expression of 34ße12 in the cholesteatoma specimens. The reported pattern was also different. We demonstrated a full-thickness expression of 34ße12 even in control specimens, supporting the hypothesis of bony EAC skin and tympanic membrane as the pathogenetic source of acquired cholesteatomas as suggested by Wittmaack (retraction pocket theory) [
15] and Jackler, et al. (mucosal traction theory) [
16]. Similar to our result, Olszewska and Sudhoff [
3] found basal layer expression in controls and increased suprabasal layer expression in cholesteatoma.
Our study suggests that while EAC skin is the source of cholesteatoma for maximum patients, metaplasia of middle ear mucosa as suggested by Sadé’s theory cannot be overlooked.
We could not demonstrate a proportionately high expression of any of the IHC markers in aggressive cholesteatomas (stage III patients). Juhász, et al. [
17] had found a higher expression of Ki67 in patient with destructive cholesteatomas. However, all four specimens from our patients with stage III disease showed low Ki67 immunostaining.
There are some limitations of this study. One, our specimen size was small. Second, we could not recruit a patient of stage IV disease which could have given us even more subgroups.
In conclusion, majority of cholesteatoma specimens significantly overexpress CK17, CK13, and Ki67 when compared to normal EAC skin controls, while a subset show loss of 34ße12 expression. While differential expression of these IHC markers is not an indicator of aggressiveness, as evidenced by their similar expression patterns across all stages, it provides some insight into its pathogenesis.
Acknowledgments
The project was supported by intramural project grant from the Indian Council of Medical Research (ICMR). We would like to thank Ms. Monika, a technical staff member for performing HE and immunostaining of specimens.
Fig. 1
34ße12 immunostaining in cholesteatoma specimens. A: Cholesteatoma specimen showing full-thickness immunopositivity with 34ße12 (×200). B: Cholesteatoma specimen showing absence of 34ße12 immunostaining (×200).
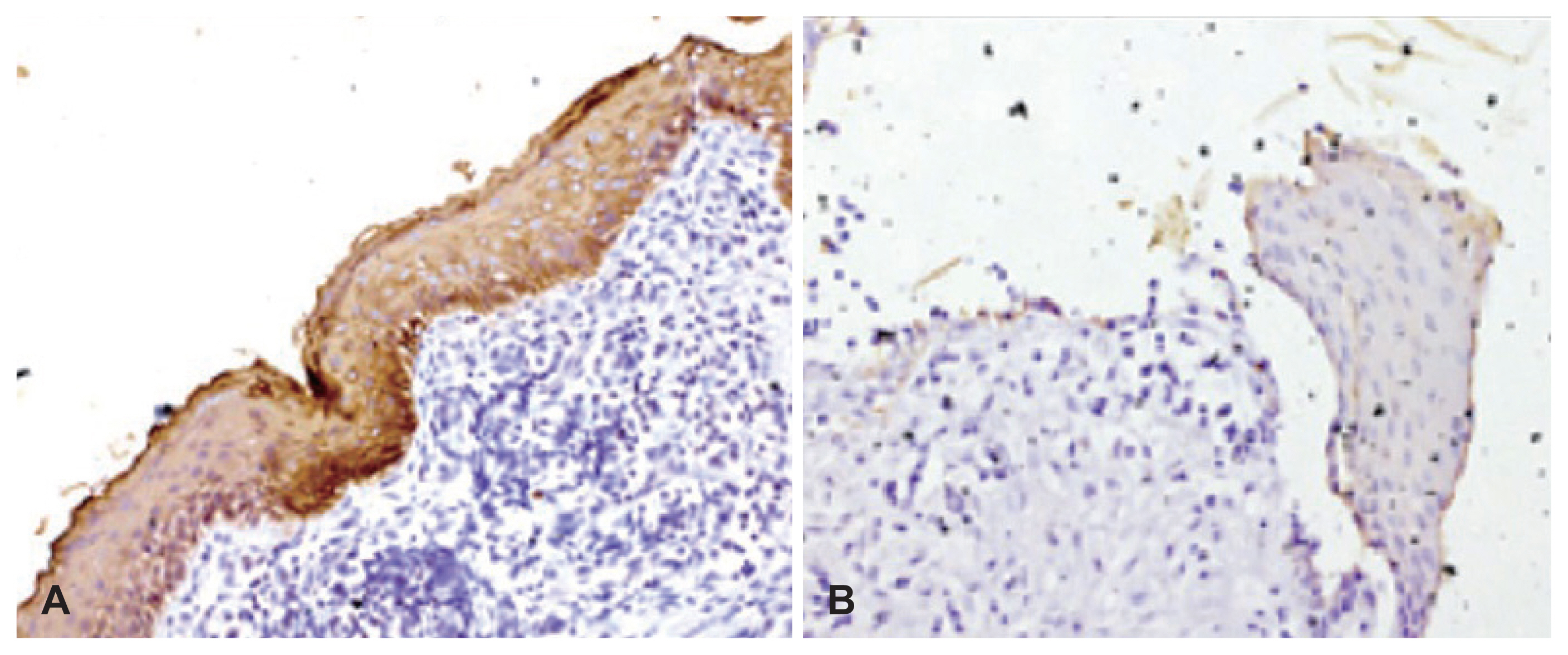
Fig. 2
CK13 immunostaining in cholesteatoma specimens. A: Cholesteatoma specimen showing full-thickness immunopositivity with CK13 (×200). B: Cholesteatoma specimen negative for CK13 (×200).
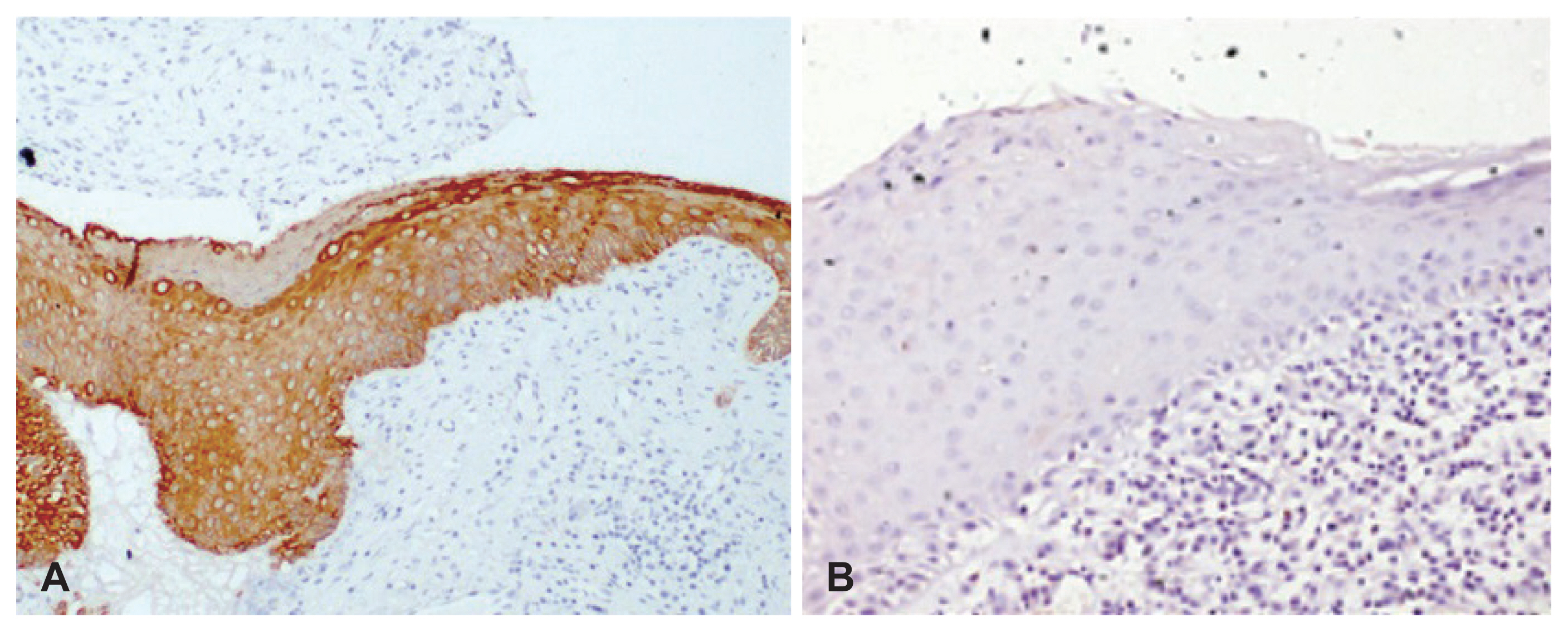
Fig. 3
CK17 immunostaining in cholesteatoma specimens. A: Cholesteatoma specimen showing immunostaining with CK17 in the suprabasal epithelial cells (×200). B: Cholesteatoma specimen showing immunostaining with CK17 in superficial epithelial cells (×200).
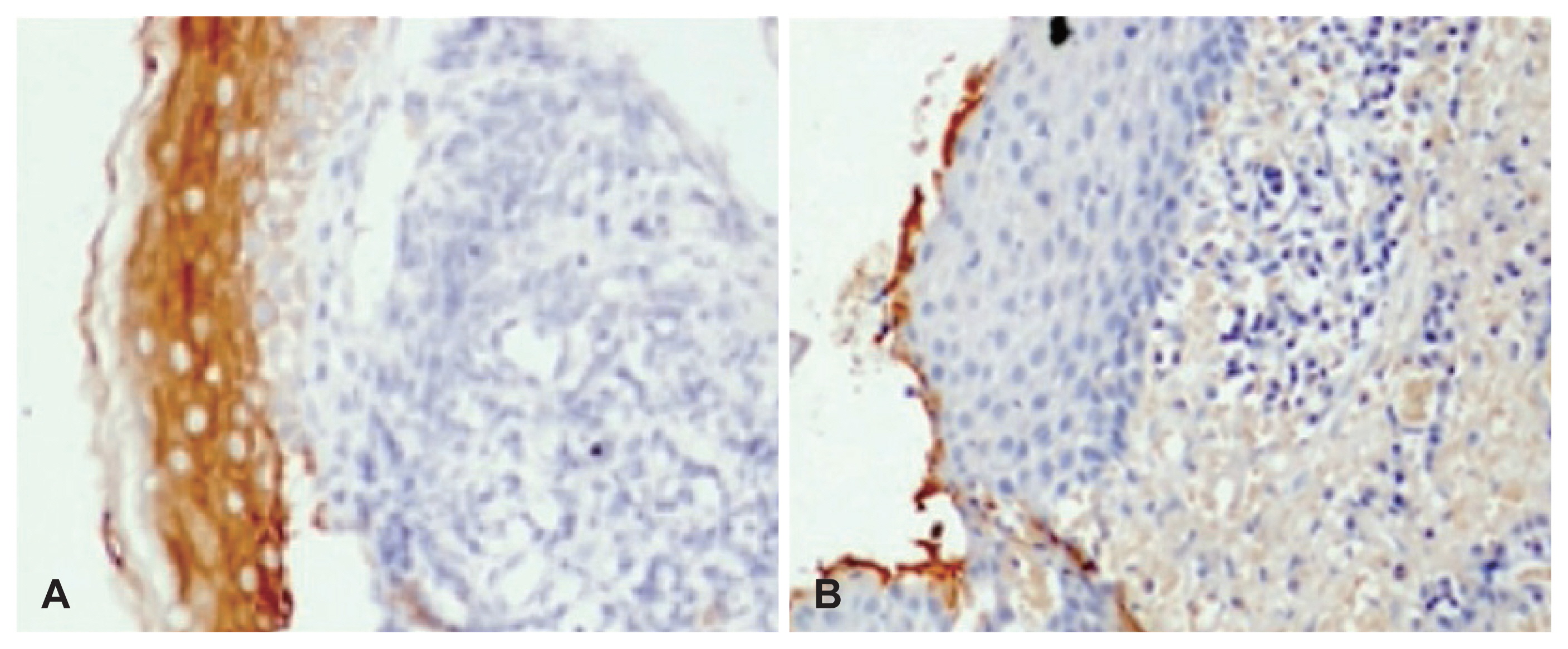
Fig. 4
Ki67 immunostaining in cholesteatoma specimens. A: Cholesteatoma specimen showing low Ki67 staining restricted to few cells in the basal layer of the epithelium (×200). B: Cholesteatoma specimen showing increased Ki67 staining extending to the upper suprabasal epithelial layers (×200).

Table 1
Clinico-demographic characteristics of the recruited subjects
|
Characteristic |
Value (n=41) |
|
Sex |
|
Female |
14 (34) |
|
Male |
27 (66) |
|
Age (yr) |
|
Mean (range) |
25 (4–68) |
|
Median |
21 |
|
≤18 |
15 (36.5) |
|
>18 |
26 (63.5) |
|
Symptoms |
|
Ear discharge |
41 (100) |
|
Hearing impairment |
41 (100) |
|
Dizziness and vertigo |
6 (14.6) |
|
Facial weakness |
0 (0) |
|
Extracranial complications |
4 (9.7) |
|
Intracranial complications |
0 (0) |
|
Type of hearing loss |
|
Conductive hearing loss |
39 (95) |
|
Sensorineural hearing loss |
0 (0) |
|
Profound SNHL |
2 (5) |
|
Clinico-radiological stage (EAONO/JOS) |
|
I |
10 (24.4) |
|
I |
27 (65.9) |
|
III |
4 (9.7) |
|
IV |
0 (0) |
|
Surgery performed |
|
Canal wall-up mastoidectomy |
2 (5) |
|
Canal wall-down mastoidectomy |
39 (95) |
Table 2
The staining patterns of cytokeratins in cholesteatoma specimens in relation to clinico-radiological staging
|
Stage |
34ße12 immunopositivity |
CK13 immunopositivity |
CK17 immunopositivity |
|
Stage I (10 specimens) |
10 |
6 |
10 |
|
Stage II (27 specimens) |
23 |
23 |
23 |
|
Stage III (4 specimens) |
4 |
3 |
3 |
|
Stage IV (0 specimens) |
- |
- |
- |
Table 3
The various subgroups of patients based on their staining pattern and their distribution as per different clinical stages
|
IHC stain |
Subgroups (based on patterns of staining) |
Number of specimens |
Stage I |
Stage II |
Stage III |
|
34ße12 |
Full thickness |
31 |
8 |
19 |
4 |
|
Negative |
10 |
2 |
8 |
0 |
|
CK17 |
Suprabasal |
28 |
9 |
17 |
2 |
|
Superficial only |
10 |
1 |
8 |
1 |
|
Negative |
3 |
0 |
2 |
1 |
|
CK13 |
Full thickness |
8 |
1 |
7 |
0 |
|
Patchy |
23 |
5 |
15 |
3 |
|
Negative |
10 |
4 |
5 |
1 |
|
Ki67 |
≤3 layers |
28 |
7 |
17 |
4 |
|
>3 layers |
9 |
3 |
6 |
0 |
|
Negative |
1 |
0 |
1 |
0 |
REFERENCES
1. Indian Council of Medical Research New Delhi. ICMR Multicentric National Task Force Project “Prevalence and Etiology of Hearing Impairment” Manual for Capacity Building and Monitoring [Internet] New Delhi: ICMR;2020 [cited 2022 Nov 2022]. Available from:
https://hearing.icmr.org.in/images/pdf/ICMR_Manual.pdf
.
3. Olszewska E, Sudhoff H. Comparative cytokeratin distribution patterns in cholesteatoma epithelium. Histol Histopathol 2007;22:37–42.

7. Yung M, Tono T, Olszewska E, Yamamoto Y, Sudhoff H, Sakagami M, et al. EAONO/JOS joint consensus statements on the definitions, classification and staging of middle ear cholesteatoma. J Int Adv Otol 2017;13:1–8.


8. Sikka K, Sharma SC, Thakar A, Dattagupta S. Evaluation of epithelial proliferation in paediatric and adult cholesteatomas using the Ki-67 proliferation marker. J Laryngol Otol 2012;126:460–3.


9. Huisman MA, De Heer E, Grote JJ. Cholesteatoma epithelium is characterized by increased expression of Ki-67, p53 and p21, with minimal apoptosis. Acta Otolaryngol 2003;123:377–82.


11. Lasminingrum L, Mahdiani S, Riana D. Association of Ki-67 level on bone destruction in chronic suppurative otitis media patients with cholesteatoma. Syst Rev Pharm 2021;12:644–9.
12. Sanli A, Tezer I, Paksoy M, Aydin S, Hardal U, Ozdemir NB. Evaluation of Ki-67 expression in recurrent cases of cholesteatoma. Kulak Burun Bogaz Ihtis Derg 2007;17:65–9. Turkish.
13. Akdogan V, Yilmaz I, Canpolat T, Ozluoglu LN. Role of Langerhans cells, Ki-67 protein and apoptosis in acquired cholesteatoma: prospective clinical study. J Laryngol Otol 2013;127:252–9.


14. Sadé J, Babiacki A, Pinkus G. The metaplastic and congenital origin of cholesteatoma. Acta Otolaryngol 1983;96:119–29.


15. Wittmaack K. Wie entsteht ein genuines cholesteatom? Archiv für Ohren-, Nasen- und Kehlkopfheilkunde 1933;137:306–32.


16. Jackler RK, Santa Maria PL, Varsak YK, Nguyen A, Blevins NH. A new theory on the pathogenesis of acquired cholesteatoma: mucosal traction. Laryngoscope 2015;125(Suppl 4):S1–14.

17. Juhász A, Sziklai I, Rákosy Z, Ecsedi S, Adány R, Balázs M. Elevated level of tenascin and matrix metalloproteinase 9 correlates with the bone destruction capacity of cholesteatomas. Otol Neurotol 2009;30:559–65.















